Have you ever wondered what the difference between a compound vs. element was? It can be a complicated topic that begins with understanding atoms.
An atom is the smallest substance that can exist. It is a fragment of a substance that can no longer be broken down on a chemical level. Atoms have a nucleus made of protons and neutrons. Small, negatively-charged particles known as electrons orbit the nucleus. Protons have a positive charge.
Now that you have a foundation of chemical knowledge, understanding compounds versus elements will come easier. Ready to learn more? Keep reading below!
What Is an Element?
Elements are groups of atoms that come together to build a substance. You may recognize the term from the periodic table. In the periodic table, elements are organized, and some may even be familiar, such as oxygen, hydrogen, and helium.
However, an element is a pure chemical substance containing only one type of atom. If you’re dealing with an element like oxygen, then it will only be made up of oxygen atoms.
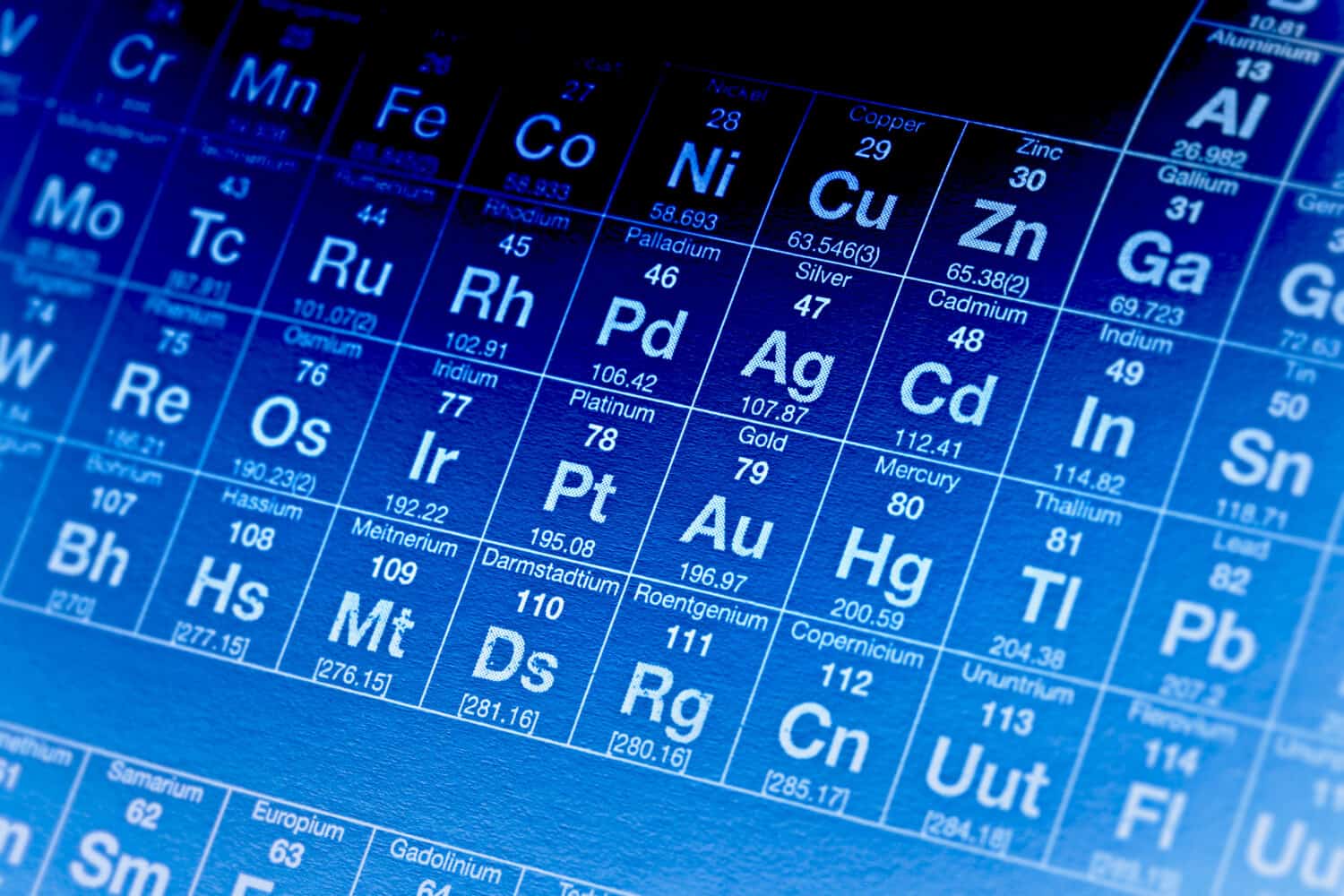
In compounds vs. elements, elements have only one atom type and can’t be broken down as a compound can.
©isak55/Shutterstock.com
Example of Elements
The entire periodic table is full of examples of elements. Many of these, such as oxygen, hydrogen, and helium, have been mentioned above. Other examples of elements, however, include silver, argon, and sodium.
What Is a Compound?
Compounds are substances that are made up of different elements. Whereas a single element will only have atoms that are associated with that element, compounds can include atoms from many different elements. As a result, these combine together to create new substances.
Unlike elements, which can only be broken down into those individual atoms, compounds can be broken down into individual elements. Additionally, more complex compounds can even be broken down into other compounds before further breaking down into elements and atoms.

Salt is a common compound in our daily lives.
©HandmadePictures/Shutterstock.com
Example of Compounds
One of the most well-known compounds in the world is water. Water has the scientific notation of H2O. This means that it is made up of two hydrogen molecules and one oxygen molecule. Other examples include table salt (NaCl) and hydrogen peroxide (H2O2).
In conclusion, we interact with compounds in our everyday lives. In addition to table salt and water, there’s sugar (C12H22O11) and vinegar (C12H22O11). Lastly, your kitchen cabinets, medicine cabinets, and pantry probably have even more compounds.
The photo featured at the top of this post is © isak55/Shutterstock.com
Thank you for reading! Have some feedback for us? Contact the AZ Animals editorial team.






