Elements are pure substances that cannot be broken down into other substances. The elemental atoms contain important subatomic particles called electrons, neutrons, and protons. The way that these subatomic particles interact within each element affects the size of its atom. Curious to learn which element has the largest atomic radius? Keep reading to find out!
What is an Atomic Radius?
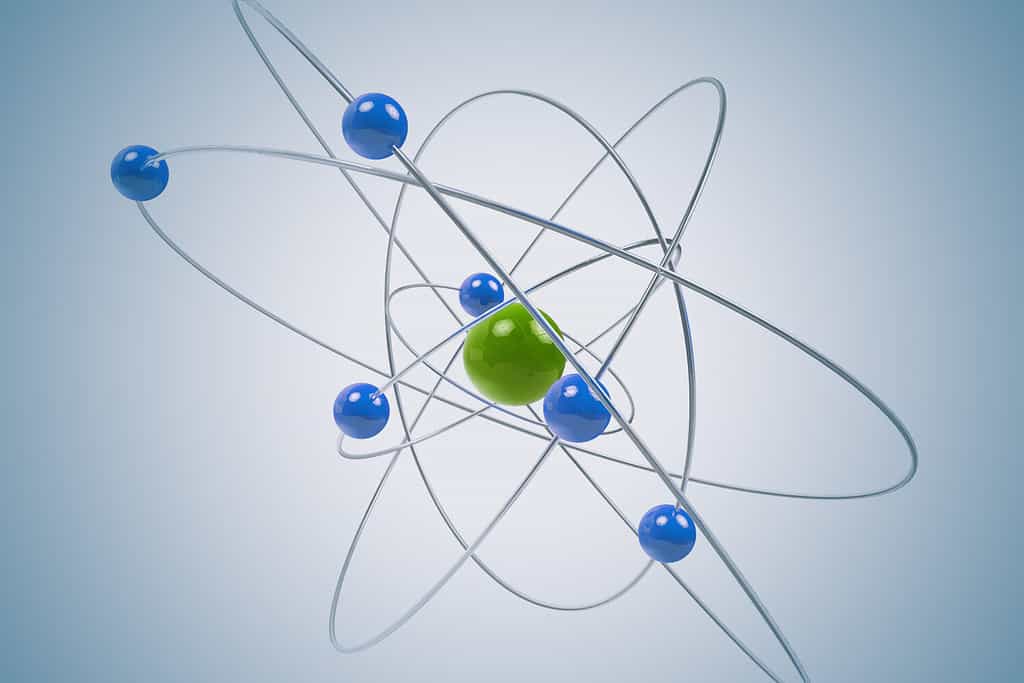
The atomic radius is the distance from the nucleus to the edge of the electron cloud in an atom.
©Dabarti CGI/Shutterstock.com
The atomic radius of an element is the distance from the nucleus of its atom to the outermost edge of the atom’s electron cloud. It approximates an atom’s size. An electron cloud is the area of negative charge in an atom created by the electrons orbiting the nucleus.
It’s not possible to directly measure the distance between an electron and its nucleus. Instead, the distance between the nuclei of two bonded atoms of the same element is divided in half. This approximates the desired atomic radius of one atom.
Technically, according to quantum mechanics, an atom is not a definite size at any given time. Also, all of the atoms in a single element will not be the same size all at once.
This is due to individualized factors affecting each atom differently as each atom’s immediate environment is not a uniform experience. Regardless, most basic chemistry concepts are organized around the easy-to-digest idea that the atoms making up particular elements are the same size.
What Element Has the Largest Atomic Radius?

Francium is the element with the largest atomic radius on the Periodic Table.
©Ekaterina_Minaeva/Shutterstock.com
Francium is the element with the largest atomic radius according to the Periodic Table. However, in practice, it’s hard to verify francium’s size because it’s such a volatile element. This element is so unstable that there is no known stable form of francium known to man.
When exposed to almost anything, francium rapidly decays. Its most stable form has a half-life of around 22 minutes and there is only about an ounce of this element anywhere in the Earth’s crust. What little naturally exists is found in uranium deposits as a byproduct of that element’s decay process.
Francium has no real value outside of scientific intrigue and the francium used by scientists is created in laboratories as a byproduct of controlled nuclear reactions. No human has ever looked at this element with the naked eye because not enough of the element exists in one place to physically see it.
What is known about the elusive element Francium is that it has an atomic number of 87. It also has an atomic weight of 223 and a melting point of about 81 degrees Fahrenheit.
Does the Periodic Table Order Elements by Atomic Radius?
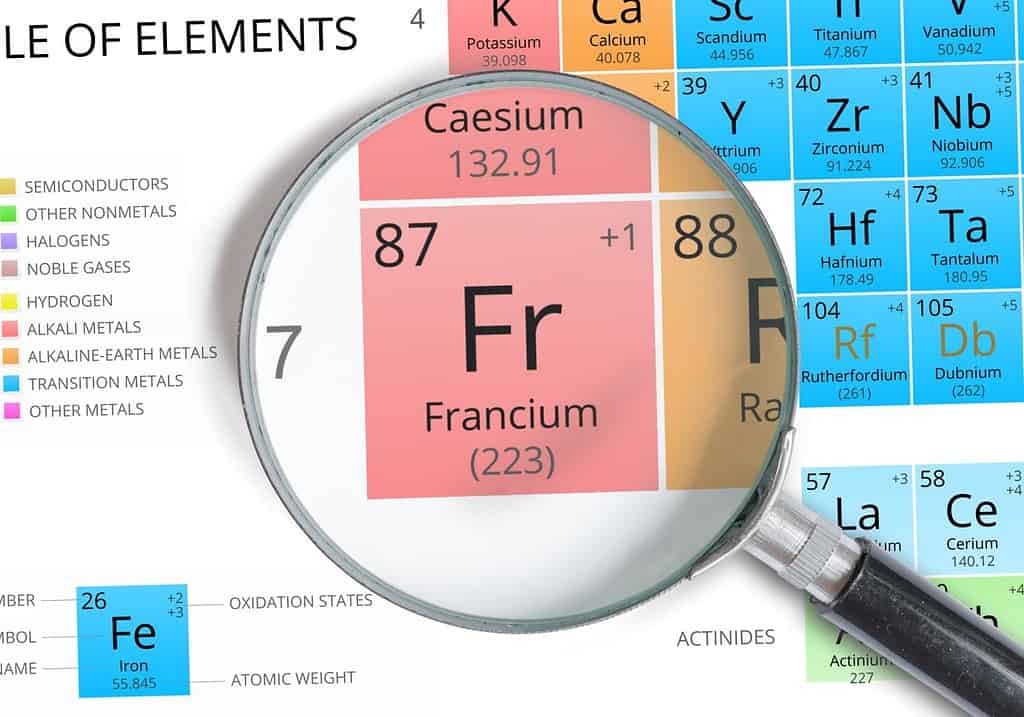
Francium is the element in the bottom left corner of the Periodic Table.
©vchal/Shutterstock.com
Yes, the Periodic Table orders elements by atomic radius. The element in the top right corner has the smallest atomic radius, while the element in the bottom left corner has the largest atomic radius.
When looking at the horizontal rows on the Periodic Table, called “across the period,” the elements on the left of the row have a larger atomic radius than those on the right. That’s because the elements on the right of the periodic table have more protons in their nucleus. Because these protons pull electrons closer to them, the radius of an atom is smaller if there are more protons to pull on outlying electrons.
When looking at vertical columns on the periodic table, known as “down the group,” the elements on the bottom have the largest atomic radii. This is because the elements toward the bottom of the table are subjected to electron shielding.
Electron shielding means that an element’s orbiting electrons interact with each other in such a way that the protons lose their grip on the outer electrons. These loosely held outer electrons increase the element’s atomic radius since they aren’t orbiting closer to the core.
What Group of Elements Has the Largest Atomic Radius?

Lithium is an alkali metal in Group 1A on the Periodic Table with a large atomic radius.
©iStock.com/nemoris
Group 1A, known as the alkali metals, has the largest atomic radii on the Periodic Table. Every element in this group has a single electron in its outermost orbital and this electron experiences minimal pull by the nucleus. As a result, it has an extremely wide orbit which results in large electron clouds and the largest atomic radii of any of the known elements.
The 6 alkali metals in Group 1A are:
- Li: Lithium
- Na: Sodium
- K: Potassium
- Rb: Rubidium
- Cs: Cesium
- Fr: Francium
As the size of the elements become bigger farther down the group, these reactive metals become more volatile. That’s because their widening orbits also mean that their loosest electron is progressively easier to remove. As a result, it doesn’t take much to set pure samples of these elements off.
When exposed to water, lithium dramatically fizzles the liquid. Potassium violently reacts to water while igniting the hydrogen gas released by their contact. Cesium and Rubidium explode when they are exposed to water. Francium is never added to water in scientific studies as the explosion potential is huge.
Cesium: Does Cs Have the Largest Atomic Radius?

The outermost electron in francium atoms spins so fast that it’s technically smaller than cesium.
©alexnako/Shutterstock.com
Yes, cesium technically has the largest atomic radius if orbital shells as defined by classical mechanics are not the standard by which to define an atom’s radius. While Francium has 7 electron shells as opposed to the 6 possessed by cesium, the behavior of Francium’s outer electron makes this atom exceptionally small in size.
The speed of the outer electron orbiting francium’s nucleus is intense and this electron travels at nearly the speed of light. Because it spins so quickly, the electron’s mass is more than it is when it’s stationary according to the relativistic effects in quantum mechanics.
This increase in mass causes more internal pull toward the nucleus as a larger mass produces a bigger charge. This bigger charge is more attractive to the protons in the nucleus.
The francium atom’s radius shrinks as the outermost electron is pulled in tighter. This size reduction is dramatic enough in francium that cesium is technically larger.
Which Element Has the Largest Atomic Number?
Oganesson has the largest atomic number at 118. An atomic number above the element’s symbol on the Periodic Table represents the number of positively charged protons in the nucleus of an atom. It also represents the number of counterbalancing electrons needed to create a stable atom.
In the early twenty-first century, scientists created oganesson in a laboratory for the first time. This makes it a synthetic chemical element. Even though it is not an element that exists naturally on Earth, it is one of 24 elements scientists have manipulated into existence in nuclear reactors.
The photo featured at the top of this post is © vchal/Shutterstock.com
Thank you for reading! Have some feedback for us? Contact the AZ Animals editorial team.






