Metal has a lustrous surface that shines brilliantly when light hits it. People mine these naturally occurring minerals deep within the Earth’s crust from mineral ores. Everyone knows metals like Aluminum, Iron, and Magnesium, but some lesser-known metals stand out because of their exceptional strength. Strength in metals doesn’t mean just being hard or tough; it includes properties like tensile strength, compressive strength, yield strength, and impact strength. These metrics define how well a metal resists external forces, whether someone is pulling it apart, compressing it, bending it, or hitting it with sudden impacts. If a metal excels in these attributes, people hail it as ‘strong’. Today, we’ll explore the ten strongest metals in the earth’s crust and discuss their unique features.
The Essence of Metal Strength

Metal used in building bridges has to maintain its strength.
Image: ThePremise, Shutterstock
©ThePremise/Shutterstock.com
Before you dive into the list, it’s essential to understand what makes a metal ‘strong’. Strength isn’t a one-dimensional property; it encompasses several attributes:
- Tensile Strength: The resistance of a metal to breaking under tension.
- Compressive Strength: A metal’s capacity to withstand being compressed or squeezed.
- Yield Strength: The point at which a metal begins to deform permanently.
- Impact Strength: A measure of how well the metal can resist fracturing when subjected to sudden impacts.
These properties are determined by the arrangement of atoms and the nature of metallic bonding. The tighter and more ordered these atoms, the harder and stronger the metal.
1. Tungsten

Consequently, it isn’t surprising that this metal finds its place in sectors ranging from aerospace to the manufacture of incandescent light bulb filaments.
©Alextov/iStock via Getty Images
Tungsten boasts the highest melting point of all metals, a staggering 3422°C (6192°F). This attribute alone makes it invaluable in applications that demand endurance against extreme temperatures. Consequently, it isn’t surprising that this metal finds its place in sectors ranging from aerospace to the manufacture of incandescent light bulb filaments. When considering its atomic weight of 183.84 u, one might be compelled to associate this heaviness with its inherent strength.
However, it’s not just about weight; it’s the structure and bonding of its atoms that grant tungsten its notable toughness. Its resilience has led to its use in various applications, from making bullets and missiles to its role in electrical and electronics sectors. The diversity in its usage is a testament to its unparalleled properties.
2. Steel

With a melting point of 1371°C (2500°F), steel offers durability, which is essential for many industrial applications.
©SimoneN/iStock via Getty Images
Steel is a widely used alloy predominantly made of iron, that stands out due to its strength and adaptability. With a melting point of 1371°C (2500°F), steel offers durability, which is essential for many industrial applications. It’s commonly found in construction, particularly in the creation of railroads, roads, and other infrastructure components.
Given that steel is an alloy, it doesn’t possess a distinct atomic weight like pure elements. Instead, its strength is derived from the combination of iron with a small amount of carbon and sometimes other elements. This mix ensures that steel maintains its form under pressure and is resistant to wear and tear. Its versatility is further highlighted by its use in various sectors, from the manufacturing of appliances to vehicle production. The range of applications for steel is broad, underscoring its fundamental role in modern industry and infrastructure.
3. Chromium

Chromium is often used for electroplating to ensure that metals remain rust-free.
Image: BeataGFX, Shutterstock
©BeataGFX/Shutterstock.com
Chromium is a metal recognized for its hardness and its ability to resist tarnishing. With a melting point of 1907°C (3465°F), it stands as a testament to endurance, particularly when exposed to high temperatures. This high resistance to heat makes it a valuable asset in many manufacturing processes. Considering its atomic weight of 51.96 u, chromium’s strength is not just about its mass. The inherent properties of chromium, including its high corrosion resistance, give it an edge in many applications.
It is this resistance that has made it a popular choice for electroplating, ensuring that metals remain rust-free and maintain their aesthetic appeal. Its primary use in the production of stainless steel showcases its versatility. By introducing chromium to steel, the resulting alloy is stronger, more resistant to rust, and often has a reflective sheen. In various industries, from automotive to construction, the presence of chromium is often a marker of quality, durability, and longevity.
4. Titanium

With an atomic weight of 47.87 u, titanium offers an impressive strength-to-weight ratio.
©RHJ/iStock via Getty Images
Titanium, associated with strength and lightness, is a metal that has significantly impacted various industries. It possesses a melting point of 1668°C (3032°F), ensuring that it retains its structure and integrity even under intense conditions. With an atomic weight of 47.87 u, titanium offers an impressive strength-to-weight ratio. This means that while it’s lighter than many metals, it doesn’t compromise on strength.
This unique balance is the reason titanium is frequently utilized in the aerospace industry, where weight and durability are paramount. Its resistance to corrosion, especially from water and chlorine, makes it a favored material for marine and underwater applications. From aircraft components to medical implants, the widespread use of titanium is a testament to its reliability and unmatched properties. In various sectors, the incorporation of titanium guarantees a blend of lightweight construction and lasting durability.
5. Iron

It has a melting point of 1536°C (2800°F), showcasing its resilience and ability to withstand various conditions.
©iStock.com/karenfoleyphotography
Iron, one of the most abundant and historically significant metals on Earth, has served as the cornerstone for many advancements in human civilization. It has a melting point of 1536°C (2800°F), showcasing its resilience and ability to withstand various conditions. With an atomic weight of 55.85 u, iron’s inherent strength and malleability have made it a staple in countless applications throughout history. It’s this combination of toughness and flexibility that has made iron the primary component in steel, an alloy that has revolutionized industries worldwide.
Its applications are vast and varied. While traditionally used for tools and weapons, today, iron, in its alloyed form, plays a crucial role in infrastructure – from bridges to buildings. It even features its magnetic properties which make it essential in electrical applications and electronics. Iron’s legacy is undeniable, serving as the backbone for many structures and tools that have shaped the course of human progress.
6. Vanadium

Vanadium enhances the performance of other metals.
Image: RHJPhtotos, Shutterstock
©RHJPhtotos/Shutterstock.com
Vanadium, while not as commonly recognized as some other metals, plays a pivotal role in enhancing the properties of other materials. With a melting point of 1910°C (3470°F), vanadium exhibits a high level of resistance to heat, making it suitable for various demanding applications. Boasting an atomic weight of 50.942 u, vanadium’s true strength lies in its capacity to fortify other metals, particularly steel. By alloying with iron, vanadium imparts increased shock and corrosion resistance to the steel, thus enhancing its durability.
Approximately 80% of the vanadium produced is used to make ferrovanadium, a steel additive. This alloyed steel finds its way into the construction of high-speed tools, gears, and engines, to name a few. vanadium is a silent enhancer, working behind the scenes to ensure other metals achieve their best performance and longevity.
7) Lutetium

Lutetium is used when heat resistance is critical.
Image: Bjoern Wylezich, Shutterstock
©Bjoern Wylezich/Shutterstock.com
Lutetium, while lesser-known in everyday applications, holds significant importance in specialized industries. With a melting point of 1663°C (3025°F), it is resilient and stands up to high temperatures, making it suitable for specific applications where heat resistance is crucial. Weighing in with an atomic weight of 174.96 u, lutetiums properties go beyond its heft. One of its notable uses is in petroleum production, where it acts as a catalyst, aiding in refining processes that are crucial for fuel production. Apart from its industrial applications, lutetium has potential uses in medicine, especially in cancer treatments. Its ability to target certain types of tumors has made it a subject of interest in medical research. In specialized sectors, lutetium is a valuable asset, showcasing the vast potential of metals in both traditional industries and cutting-edge research.
8) Zirconium
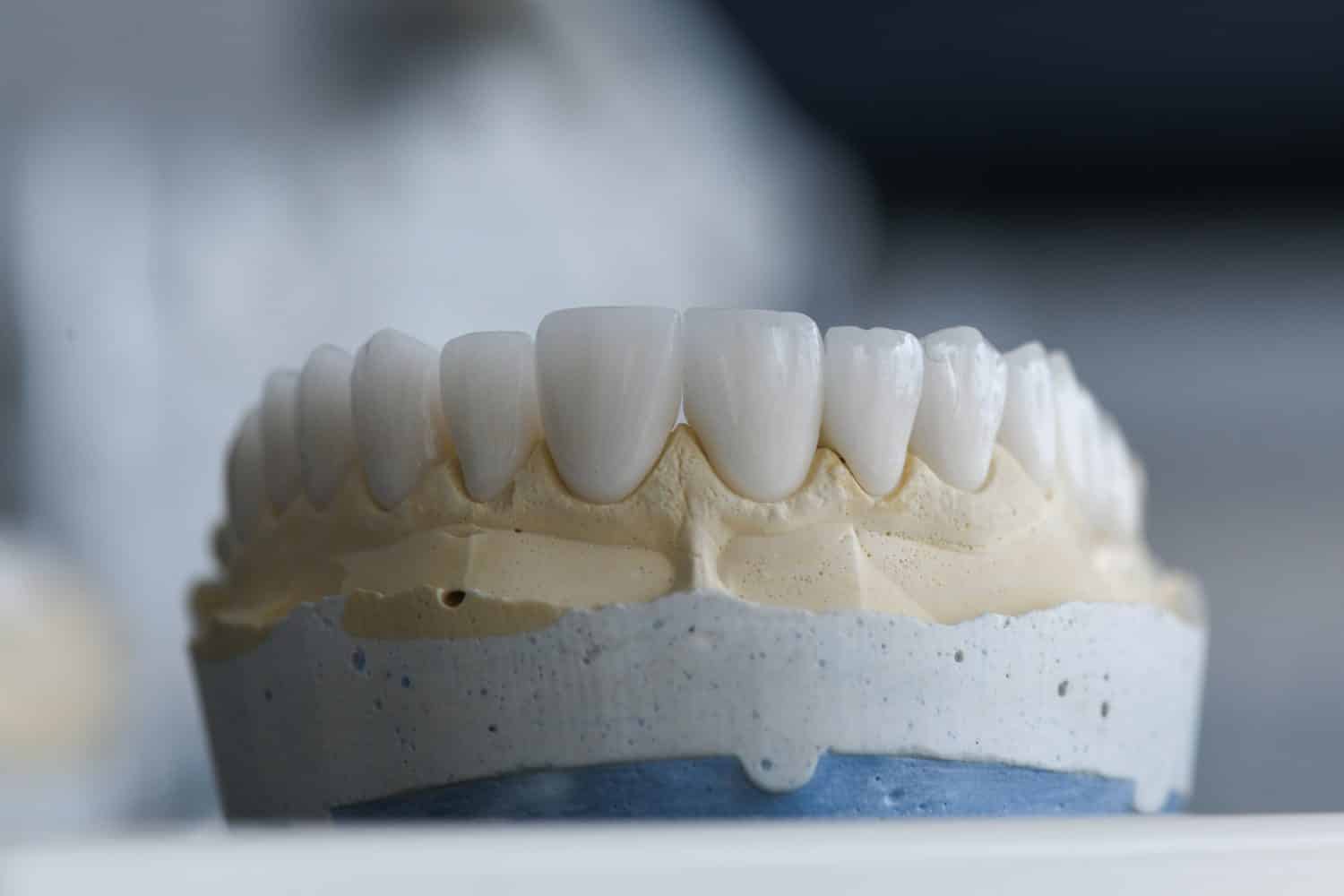
Zirconium is used to make crowns and dental implants.
Image: SerdarOmer Atmaca, Shutterstock
©SerdarOmer Atmaca/Shutterstock.com
Zirconium, distinguished by its lustrous appearance and resistance to corrosion, plays a crucial role in applications where stability is paramount. It has a melting point of 1850°C (3362°F), demonstrating its ability to maintain integrity in high-temperature scenarios. With an atomic weight of 91.22 u, Zirconiums resistance to heat and corrosive environments makes it a prized material in specific industries. One of its most significant applications is in nuclear power stations, where zirconium alloys are used as fuel cladding. This usage underscores the metal’s ability to resist the corrosive effects of high-temperature water and steam, ensuring safe operations within reactors. Zirconium also finds applications in various fields ranging from medical implants, due to its biocompatibility, to its use in creating heat-resistant ceramics.
9) Osmium

Osmium is one of the heaviest metals on earth.
Image: LuYago, Shutterstock
©LuYago/Shutterstock.com
Osmium stands out as one of the densest metals on Earth, a trait that directly contributes to its unique set of applications. It has a melting point of 3000°C (5400°F), one of the highest among the naturally occurring elements, emphasizing its resistance to extreme heat. With an atomic weight of 190.2 u, osmium’s impressive density is not its only distinguishing feature. This metal’s hardness, especially when alloyed with other elements like platinum or indium, makes it invaluable for applications requiring wear resistance. One of its primary uses is in the production of electrical contacts and filaments, where its durability ensures long-lasting performance. Due to its reflective properties, osmium tetroxide has been utilized in microscopy as a staining agent.
10) Tantalum

Tantalum is often used to make wire that resists corrosion and high heat.
Image: beejung, Shutterstock
©beejung/Shutterstock.com
Tantalum, known for its high melting point and remarkable resistance to corrosion, is a metal that performs exceptionally well under challenging conditions. With a melting point of 3,017°C (5,462°F), tantalum stands firm even when exposed to extreme heat. Possessing an atomic weight of 180.94 u, tantalum is not only durable but also versatile. One of its standout properties is its remarkable resistance to corrosion, even against acids. This resistance is why tantalum is frequently used in chemical processing equipment, ensuring that processes remain uncontaminated and efficient.
Its high melting point and anti-corrosion properties make it a preferred metal for alloying, enhancing the strength and heat resistance of the resulting alloy. Additionally, it finds applications in electronics, particularly in capacitors, due to its ability to hold a charge effectively. Tantalum’s unique properties make it indispensable in industries where strength, heat resistance, and anti-corrosion are essential attributes.
Comparison Chart of the Strongest Metals
| Rank | Type of Metal | Alternative Use | Atomic Weight | Melting Point |
|---|---|---|---|---|
| #1 | Tungsten | Filaments in light bulbs | 183.84 u | 3422°C / 6192 °F |
| #2 | Steel | Production of surgical instruments | n/a | 1371°C / 2500°F |
| #3 | Chromium | Electroplating to prevent metal corrosion | 51.96 u | 1907°C / 3465°F |
| #4 | Titanium | Making underwater submersibles due to corrosion resistance | 47.87 u | 1668°C / 3032°F |
| #5 | Iron | Crafting historical weapons and armor | 55.85 u | 1536°C / 2800°F |
| #6 | Vanadium | Used in superconducting magnets | 50.942 u | 1910°C / 3470°F |
| #7 | Lutetium | In refining petroleum processes | 174.96 u | 1663 °C / 3025°F |
| #8 | Zirconium | Crafting heat-resistant ceramics | 91.22 u | 1850°C / 3.362°F |
| #9 | Osmium | Added to platinum or indium to make them harder | 190.2 u | 3000°C / 5,400°F |
| #10 | Tantalum | Crafting specialized surgical instruments due to its non-reactive properties | 180.94 u | 3,017°C / 5462°F |
Thank you for reading! Have some feedback for us? Contact the AZ Animals editorial team.








