Hydrochloric acid is one of the most common acids. Chemists use it frequently in the lab for a wide variety of reactions. It is a strong acid and has a pH generally between about 1 and 2, depending on its concentration. Hydrochloric acid has many uses in a wide variety of industries. This article will help you discover the molar mass of hydrochloric acid and provide you with a look at how it compares to other similar compounds.

Hydrochloric acid is one of the most common acids, with uses in the laboratory and many industries.
©Kittisak Kaewchalun/iStock via Getty Images
The Molar Mass of Hydrochloric Acid
Hydrochloric acid is a pure substance. A pure substance has the same chemical composition throughout, including even the smallest particles. All the elements on the periodic table, as well as all defined chemical compounds, qualify as pure substances. The smallest particles in elements are atoms, whereas molecules are the smallest particles of a compound. The molar mass of each pure substance influences the physical and chemical properties of the substance. The molar mass of hydrochloric acid is 36.46 grams per mole.
So, what is the molar mass of a substance? The molar mass is defined as the mass of one mole of a pure substance expressed in grams. Great, that’s simple, but what is a mole? A mole is just a unit of measurement. Just like one dozen equals 12 of any given thing, one mole equals 6.022 × 1023 of anything. You could have a mole of doughnuts, or a mole of dimes, but in reality, we usually measure atoms or molecules in terms of moles. We call that giant number, approximately 6.022 × 1023, Avogadro’s number or Avogadro’s constant. This important constant, named for the Italian scientist, Amadeo Avogadro, is always the same. It is essential to understanding chemistry and how the world works.
Molar Mass vs. Atomic Mass
We can determine the molar mass of an element or compound by first learning its atomic mass. Look for the atomic mass of an element on the periodic table. This value, usually listed just below or to the side of the chemical symbol, should be noted on the table’s key. Scientists determine the atomic mass of each element by taking account of all the known isotopes of the element. They average the masses of these isotopes, weighted by their proportion as found in nature, to find the atomic mass for the element.
Each element on the periodic table has a fixed number of protons, and most have a variable number of neutrons. Atoms of an element with different numbers of neutrons are known as isotopes. The number of protons in an atom’s nucleus equals the atomic number of that element. The number of neutrons determines which isotope of the element the particular atom represents.
Every individual proton or neutron has a value of one atomic mass unit. The mass of any number of electrons in the atom’s orbitals is so tiny that it can be disregarded. Therefore, the atomic mass of any isotope equals the number of protons it has plus the number of neutrons.
An Example of How Isotopes Work
We must understand isotopes in order to learn about molar mass. Let’s examine the element, carbon, to learn more about how isotopes work. Carbon has only three different isotopes: carbon-12, carbon-13, and carbon-14. The lightest isotope, carbon-12, has six protons and six neutrons. Therefore, its atomic mass equals 12. Meanwhile, carbon-13 has six protons and seven neutrons, which results in an atomic mass of 13. And the heaviest isotope, carbon-14, has six protons and eight neutrons. That gives it an atomic mass of 14. The average of these three numbers is 13, but that is not equal to the calculated atomic mass of carbon, which is 12.011 atomic mass units. Because carbon-12 isotopes far outnumber carbon-13 and carbon-14 in nature, the average must be weighted. Therefore, considering the much higher representation of carbon-12, the atomic mass skews heavily toward 12.
Calculating the Molar Mass of Hydrochloric Acid
As you learned, the molar mass of an element simply equals its atomic mass expressed in terms of grams per mole. The mass of one mole of atoms of an element equals the atomic mass of that element, but in grams.
This same concept applies to compounds, except that each molecule of a compound contains two or more atoms of the same or different elements. To find the atomic mass of a compound, we must add up the atomic masses of all the atoms in the molecule. The total equals the compound’s atomic mass.
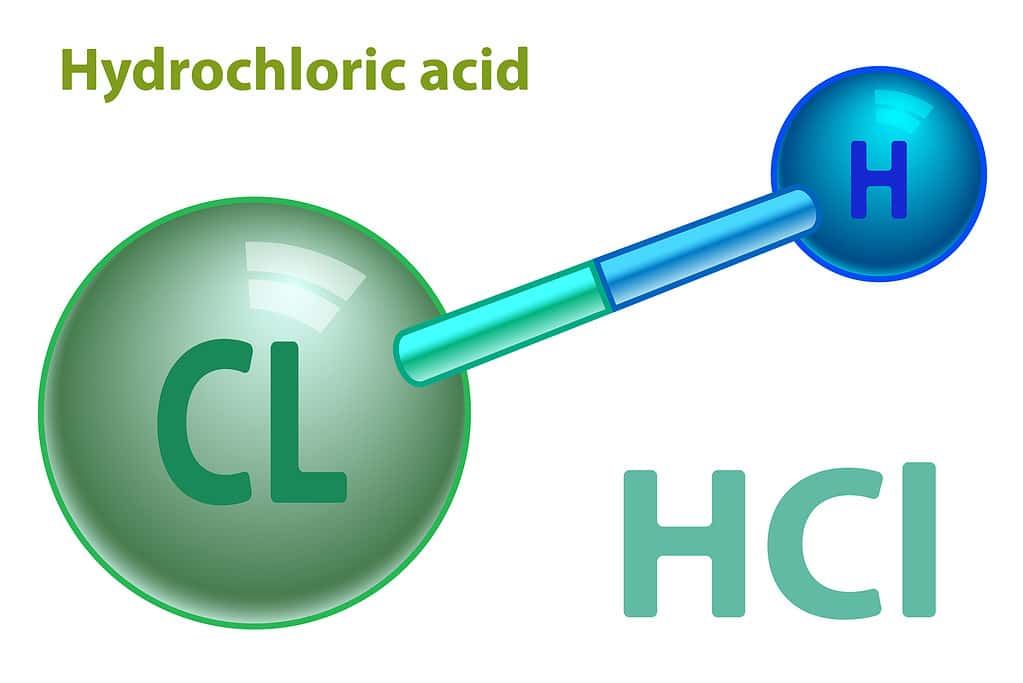
Hydrochloric acid, HCl, has just two atoms in each molecule. It has one chlorine atom bonded to one hydrogen atom. Therefore, one mole of hydrochloric acid molecules has one mole of chlorine atoms and one mole of hydrogen atoms. To find the mass of one mole of chlorine, just convert the atomic mass of chlorine to grams. One mole of chlorine has a mass of 35.45 grams. And one mole of hydrogen has a mass of 1.008 grams. Adding the mass of the two atoms in this compound gives a total of 36.46 grams as the molar mass of hydrochloric acid.
| Calculating Molar Mass of Hydrochloric Acid |
|---|
| Simplified chemical equation for hydrochloric acid: H + Cl → HCl |
| 1 mole of hydrogen + 1 mole of chlorine → one mole of hydrochloric acid |
| 1.008 grams H/mole + 35.45 grams Cl/mole → 36.46 grams HCl/mole |
Atomic Structure of Hydrochloric Acid
Hydrochloric acid is a molecular compound made up of just two atoms: hydrogen and chlorine. When hydrogen and chlorine combine as a gas, the compound is called hydrogen chloride. But when this gas dissolves in water, we call the resulting solution hydrochloric acid.
Chlorine belongs to Group 17 on the periodic table. Group 17 contains the halogens: fluorine, chlorine, bromine, iodine, etc. The members of this group reside just one place away from Group 18, the noble gases. They need just one electron to reach their desired, stable electron configuration. They form halide ions with a -1 charge, including the chloride ion, Cl–.
Hydrogen naturally occurs as a diatomic gas, with each hydrogen atom covalently bonded to another. Traveling alone, it forms a single hydrogen ion with a +1 charge. This hydrogen atom easily bonds to any ion with a -1 charge, such as the chloride ion, to form a molecule with a neutral charge.
Hydrochloric acid is considered a strong acid because the covalently bonded hydrogen and chlorine atoms dissociate, or break apart into ions, almost completely in water. That makes hydrochloric acid very useful in a wide array of chemical processes.
Hydrochloric Acid Configuration
The atoms in hydrochloric acid arrange themselves in a linear structure with a 180 degree bond angle, just like any other diatomic molecule. They form a bond known as a polar covalent bond. Covalent means that they share an electron so that both atoms have a stable configuration with filled outer orbitals at least part of the time. Having a polar covalent bond means that the electron is unequally shared. One of the atoms in the pair gets the extra electron more of the time, giving it a partial negative charge, while the other atom has a partial positive charge. In this case, chlorine has a greater attraction for the electron, but not enough to completely take away the electron. So, this linear molecule ends up polarized; it is more negative on the chlorine side and more positive on the hydrogen side.
Hydrochloric acid is a polar covalent molecule made of hydrogen and chlorine.
©AlexanderZam/iStock via Getty Images
Hydrogen Donor
Hydrochloric acid has an important use as a hydrogen donor. When it breaks down, or dissociates in water, it provides hydrogen ions that can be picked up by other chemicals to make new compounds or polyatomic ions.
(Hydrochloric acid) HCl → (Chlorine ion) Cl– + (Hydrogen ion) H+
One example of how this donated hydrogen ion can be used is the conversion of ammonia to the ammonium ion. Ammonium ions can then be used to make important products such as nitrogenous fertilizers.
(Ammonia) NH3 + (Hydrogen ion) H+ → (Ammonium ion) NH4+
Strong acids like hydrochloric acid provide more hydrogen ions than weak acids, because they dissociate almost completely into ions in solution.
Hydrochloric Acid in Nature
Hydrochloric acid is produced in nature, but not in easily accessed sources or in the large quantities needed for its many uses today. You are intimately familiar with one of the main natural sources of hydrochloric acid. Humans and other vertebrates have cells in their stomach that produce hydrogen and chloride ions. These combine to form hydrochloric acid, which helps the body break down food, absorb essential minerals, and destroy harmful microorganisms.
Some volcanoes also produce hydrochloric acid by spewing hydrogen chloride gas into the air, which dissolves in water in the atmosphere. This hydrochloric acid is a component of acid rain, which also typically includes sulfuric acid, H2SO4.
Hydrochloric Acid Production
Hydrochloric acid can be produced in multiple ways, including combining hydrogen and chlorine or adding sulfuric acid to sodium chloride. Most of the hydrochloric acid used in industries today comes from large scale production of other chemicals. For a long time, chemical manufacturers just released the hydrogen chloride that formed as a gas when producing other chemicals on a large scale. This gas entered the atmosphere as a waste product and caused acid rain, which was harmful to the environment. Now, much of the hydrogen chloride produced as a byproduct of other processes must be captured due to anti-pollution laws. This gas is collected over water to form hydrochloric acid, which can then be sold and used for many purposes.
Common Uses of Hydrochloric Acid
Hydrochloric acid has many different uses from the food and drug industries to oil and mining operations, and all sorts of things in between. Here are just a few of the most common uses.
Food Processing
The food industry uses hydrochloric acid in a wide variety of ways to produce many different types of foods. One of the primary uses for hydrochloric acid is the processing of corn into products such as high fructose corn syrup and corn starch. It also facilitates the manufacture of gelatin from bones, and the making of soy sauce. Artificial sweeteners and a number of food additives rely on hydrochloric acid. And various acidic products, such as sauces, tomato juice and other vegetable juices, and assorted canned foods utilize hydrochloric acid for maintaining a lowered pH level.
Drug Manufacture
The pharmaceutical industry uses hydrochloric acid to adjust the pH during the manufacture of drugs. The acid also functions as a catalyst and as a reduction agent.
Oil Drilling
Before drilling for oil, hydrochloric acid is pumped into the ground to partially dissolve the rock and improve the flow of the oil. Drillers also use hydrochloric acid to clean carbonate deposits from inside oil wells and get rid of any rust and scale on the metal.
Mining Operations
Mining operations use hydrochloric acid to treat raw ores, to extract and separate wanted minerals from mixtures, and treat the water used in mining activity. It proves very useful in stabilizing the pH when processing basic solutions, such as those containing potash.
Processing Steel and Aluminum
The steel and aluminum industries use hydrochloric acid to process metals. This acid helps to remove rust and scale from the surface of steel so it can be used to make specialized products including wire, tin, and strip steel. They also use hydrochloric acid to etch aluminum and prepare for galvanizing and other uses.
Textile Processing
Manufacturers in the textile industry use hydrochloric acid in a number of ways. This acid is essential to producing quality dyes and pigments. It helps with tanning and dying leather. And workers use it to dye and bleach different types of fabrics and to treat some kinds of wool.
Producing Calcium Chloride
We use the salt, calcium chloride, for several purposes, but the most important is probably the treatment of roads to prevent ice and blowing dust. Reacting the naturally occurring compound, calcium carbonate, with hydrochloric acid yields calcium chloride in a relatively simple process.
Dangers of Hydrochloric Acid
Hydrochloric acid is highly corrosive. The exact pH of the acid depends on its concentration in solution. The more concentrated the solution, the lower the pH, and the more acidic and corrosive the solution. Hydrochloric acid should always be handled with care. Users should practice standard lab safety, wearing goggles to avoid allowing fumes to come in contact with the eyes, and rubber gloves to avoid contact with skin. Highly concentrated hydrochloric acid should be handled under a fume hood to better protect the eyes. If this acid is swallowed, do not induce vomiting, but do seek immediate medical attention.

Hydrochloric acid is highly corrosive. Always handle it with caution.
©Kittisak Kaewchalun/iStock via Getty Images
Other Common Acids
Hydrochloric acid, with just two atoms, is one of the simplest and most common acids. This strong acid shares much in common with other acids made from hydrogen and a Group 17 halide, such as hydrofluoric acid and hydrobromic acid. The table below lists some of the most common acids, their molar mass, and a few of their interesting uses.
| Compound Name | Chemical Formula | Molar Mass | Interesting Uses |
|---|---|---|---|
| Hydrochloric acid | HCl | 36.46 g/mol | Neutralizing bases; foods; drugs; cleaning agent; leather processing |
| Hydrofluoric acid | HF | 20.01 g/mol | Precursor to refrigerants, fluorescent bulbs, drugs, etc. |
| Hydrobromic acid | HBr | 80.91 g/mol | Production of bromide compounds |
| Acetic acid | CH3COOH | 60.05 g/mol | Vinegar; antiseptic; solvent |
| Nitric acid | HNO3 | 63.01 g/mol | Precursor to fertilizers, explosives, etc. |
| Sulfuric acid | H2SO4 | 98.08 g/mol | Precursor to fertilizers, drugs, detergents, etc.; batteries |
| Acetylsalicylic Acid | C9H8O4 | 180.33 g/mol | Aspirin, drug used to treat fever and inflammation |
| Citric acid | C6H8O7 | 192.12 g/mol | Food additive; cosmetics; cleaning agent |
Conclusion
As you have learned, hydrochloric acid is one of the most common acids, made of just two atoms. Manufacturers produce this important compound easily as a byproduct of other processes. It has many important uses in a variety of industries, but due to its highly corrosive nature it must be handled with great care.
Thank you for reading! Have some feedback for us? Contact the AZ Animals editorial team.








