When we talk about understanding an ion, we’re talking about an atom or a molecule. So in that sense, it’s a very small thing. But in terms of the roles that ions play in the world around us, these teensy particles are a very big deal indeed. And when scientists began to explore and understand what ions are and how they work, that knowledge became a game changer in chemistry and physics.
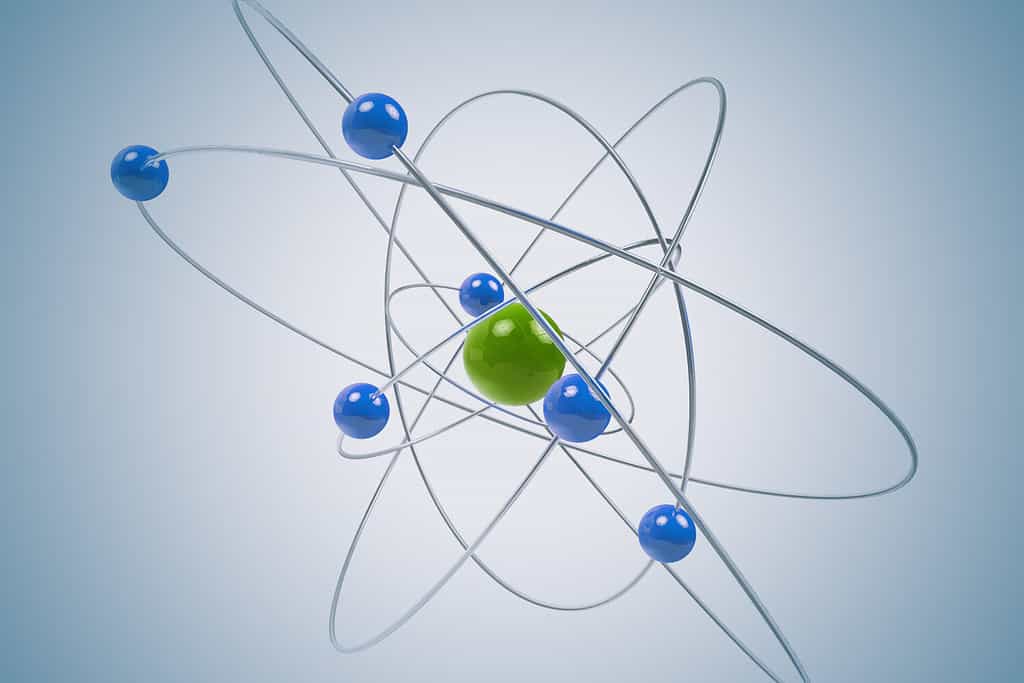
Why so negative? An atom with more electrons than protons is an anion and has a negative charge.
©Dabarti CGI/Shutterstock.com
What are Ions?
The simplest way to define an ion is this: an ion is an atom or molecule that carries an electrical charge. The term itself was coined in the 19th century by physicist and chemist Michael Faraday (a familiar name to anyone who’s pursued those scientific disciplines). Faraday and other scientists of the day observed what happened when the electrodes of a battery were placed in water. They theorized that some unknown substance was traveling from one electrode to the other. “Ion” was taken from a Greek word meaning “to go.” The term is also the base for the words “anion,” an ion with a negative charge, and “cation,” an ion with a positive charge. Those words were coined to reference which direction the unknown materials were traveling (also derived from Greek words, “an” = “up,” “cat” = “down”).
The Positive and Negative of it All
Understanding what ions are all about requires knowing how atoms are structured. So let’s review the basics. Atoms are composed of three fundamental particles: Protons are particles with a positive charge. Neutrons are particles with no charge. And electrons are particles that carry a negative charge. The charge that each proton carries and the charge that each electron carries are opposite, but equal in strength, so they cancel each other out. This means:
- An atom with the same number of protons and electrons has no charge.
- An atom with more protons than electrons will have a positive charge (it’s a cation).
- An atom with more electrons than protons will have a negative charge (it’s an anion).
- Some ions are made up of multiple atoms. These are called polyatomic ions.
An important characteristic of ions is that their opposite charges attract each other. That is, positive ions and negative ions attract each other, and this attractive force can form chemical bonds. One example of this is sodium chloride, which is the chemical name for common table salt. Sodium chloride is made up of positive sodium ions and negative chlorine ions (cations and anions, respectively, if you’re keeping track).
What Ions Get Up To
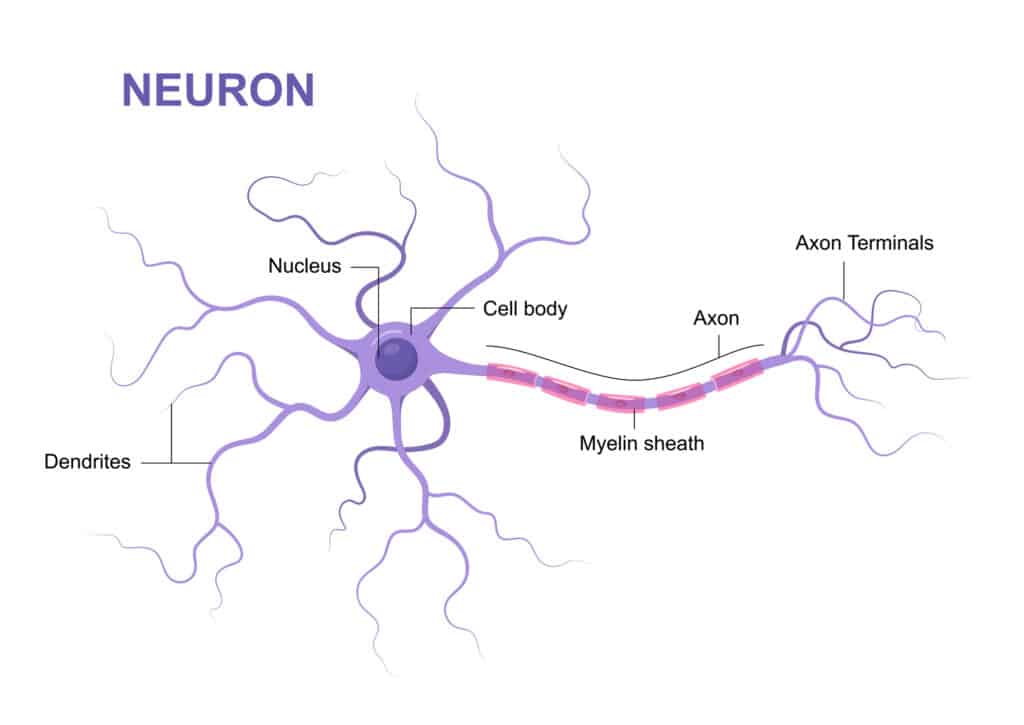
Putting the “I” in “ion:” the cells of your nervous system use ions to send electrical signals.
©iStock.com/Vitalii Dumma
Because they interact with each other and other atoms, ions take part in all kinds of chemical processes. Examples include:
Electric charge: In batteries, it’s ions that carry the electric charge and conduct electricity.
Acidity: Hydrogen cations are what make a substance acidic.
Mineral coloration: Ions, and the different wavelengths of light they absorb, are responsible for the color of different gemstones.
Your brain and nervous system: The cells of your nervous system are able to send electrical signals because their membranes are permeable to certain ions.
Free radicals: Some ions, called radicals, are highly reactive. They can even damage the cells of the body, though they may also have beneficial effects, and may help regulate tissue growth. Your body produces radicals as part of its normal metabolism, and also has natural defenses against them.
Technology: Some specialized scientific equipment, like mass spectrometers, use ions. Some air purification systems use them. The theory is that the ions will attach to airborne particles and cause them to settle out of the air. According to the Environmental Protection Agency, this won’t work very well against many indoor pollutants.
Can Ions Affect My Mood?

Get your negative ions here: waterfalls and other sources of splashing water generate anions.
©Radoslav Cajkovic/Shutterstock.com
Maybe they can!
Ions, and negative ions, in particular, have been linked to mood-boosting mental health benefits. Part of the appeal of this idea is the fact that we can find anions in all sorts of natural settings. The sun’s ultraviolet rays create them. So does the electricity discharged in a thunderstorm. Many plants generate negative ions as part of their growth process. And the physical breakup of water into droplets, like at a waterfall, or where waves break onto the seashore, produces negative ions too.
The scientific literature on this does suggest that exposure to negative ions for several hours improves symptoms of depression. In the case of seasonal depression, researchers documented effects after just 30 minutes of exposure. But reviews of ion research have found no effect of negative ion exposure on general mental health or anxiety.
It’s worth noting that devices claiming to benefit your health by producing ions have no evidence backing them up. And they may produce ozone and other substances that can irritate your lungs and airways. Fortunately, there’s no shortage of negative ions in natural settings. So you have one more reason to spend more time outdoors! Time spent near fountains, at a waterfall, creek, or beach, or even in the rain, will spray you with anions as well as water. And your house probably has a built-in negative ion generator…also known as a shower!
The photo featured at the top of this post is © Sergey Nivens/Shutterstock.com
Thank you for reading! Have some feedback for us? Contact the AZ Animals editorial team.






