The molar mass of an element or compound is a fundamental concept in chemistry. Here, we will explore the molar mass of chlorine and explore why this value is crucial in understanding the behavior of this element. Additionally, we will discuss the real-world presence of this element, how its molar mass is calculated, and how it compares to the molar masses of other elements. Let’s begin by understanding what molar mass is and why it matters in the world of chemistry.
What is Molar Mass?
Molar mass, often referred to as molecular weight or atomic mass. It represents the mass of one mole of a given substance, typically expressed in grams per mole (g/mol). It is a fundamental property of any element or compound and plays an important role in various chemical calculations. This includes stoichiometry, through which we can determine the amount of substance in a chemical reaction and understanding the composition of compounds.
To calculate the molar mass of an element, we sum up the atomic masses of all the atoms in one mole of that element. This is done using the atomic mass units (amu) from the periodic table. For compounds, you sum the atomic masses of all the atoms in one molecule of the compound. The molar mass is a critical piece of information for chemists. This is because it aids in making accurate measurements, conducting experiments as well as understanding chemical reactions.

Hydrochloric acid is one of the most common acids, with uses in the laboratory and many industries.
©Kittisak Kaewchalun/iStock via Getty Images
Molar Mass of Chlorine
Chlorine is a halogen gas known for its distinctive smell. It has the chemical symbol Cl and atomic number 17. Since it is a halogen, it plays a role in disinfection and water treatment.
The molar mass of this element (Cl) is approximately 35.453 grams per mole (g/mol). This value tells us that one mole of chlorine atoms has a mass of about 35.453 grams. In other terms, if you were to gather 6.022 x 10²³ individual atoms or molecules of this element, their combined mass would be 35.453 grams.
Why is Knowing Chlorine’s Molar Mass Important?
Understanding the molar mass of this element is vital for various reasons:
1. Chemical Reactions and Stoichiometry:
One of the primary reasons for understanding the molar mass of chlorine is its role in chemical reactions and stoichiometry. Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in a chemical reaction. By knowing the molar mass of this element, chemists can precisely determine the quantity of reactants required to produce a certain amount of products and vice versa.
For example, consider the reaction between hydrogen (H₂) and chlorine gas (Cl₂) to form hydrogen chloride (HCl). Here, one mole of hydrogen gas combines with one mole of chlorine gas to form two moles of hydrogen chloride.
To balance and calculate the reaction’s yield or the amount of reagents needed, knowing the molar mass of this element is crucial. Without this knowledge, chemists would struggle to predict the reaction’s outcome accurately.
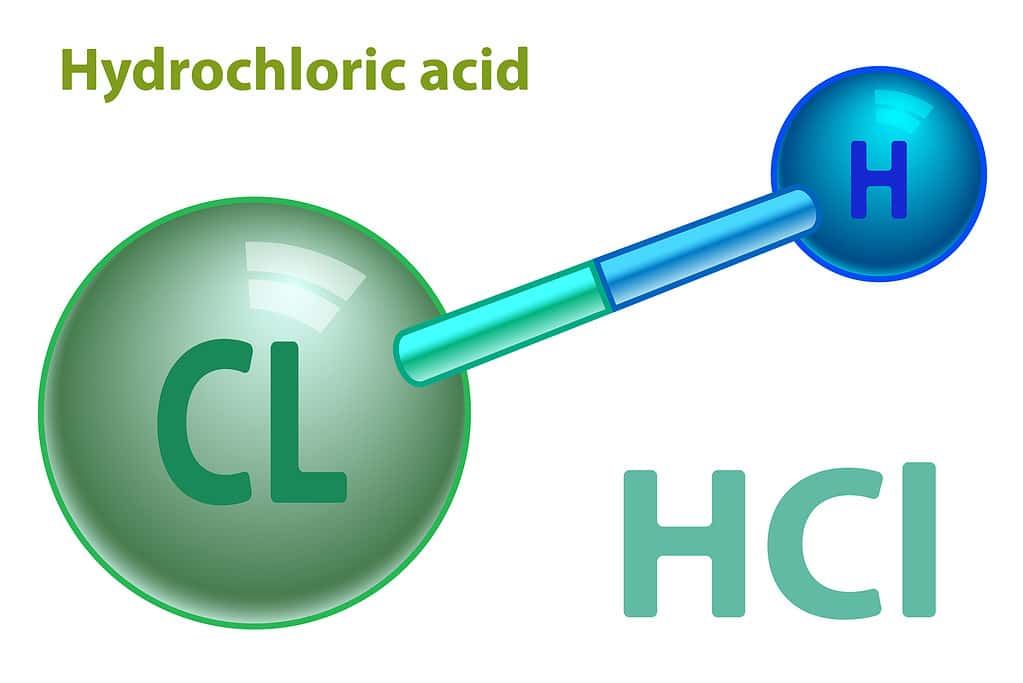
Hydrochloric acid is a polar covalent molecule made of hydrogen and chlorine.
©AlexanderZam/iStock via Getty Images
2. Formulating Compounds:
Chlorine is not just an element but also forms a component in numerous compounds. Its molar mass is vital in the formulation of various chemical compounds. This is particularly true in industries such as pharmaceuticals, where precise dosing is critical.
For instance, consider the synthesis of sodium chloride (table salt). In this reaction, two moles of sodium combines with one mole of chlorine gas to form two moles of sodium chloride.
Here, knowing the molar mass of this element is essential in determining the appropriate amount of gas needed to react with sodium. Sodium chloride, also known as table salt, is commonly used as a seasoning and food preservative.

Table salt is made up of sodium and chlorine.
©fen deneyim/Shutterstock.com
3. Analyzing Mixtures:
Analytical chemistry involves the identification and quantification of substances within mixtures. The molar mass of chlorine is a fundamental parameter in techniques like mass spectrometry. This technique relies on measuring the mass-to-charge ratio of ions to identify and quantify compounds.
In mass spectrometry, the molar mass of this element helps researchers identify chlorine-containing compounds in complex mixtures. This includes environmental samples, pharmaceutical formulations, or forensic analyses. This capability is also invaluable in fields ranging from environmental science to criminal investigations.
4. Safety Precautions:
Chlorine gas in its elemental form, however, is hazardous to human health and safety. Understanding its molar mass is essential for safety protocols and risk assessment. This is because when handling or working with this element, people must learn to be safe. Precise measurements of chlorine levels are, therefore, critical to ensure that exposure remains within safe limits, preventing potential health risks associated with chlorine gas.
5. Environmental and Industrial Applications:
Beyond its use in the lab, chlorine’s molar mass is also useful in various industrial and environmental applications. Industrialists use chlorine in water treatment to disinfect drinking water and wastewater. Proper dosing of this element, based on its molar mass, also ensures the removal of harmful microorganisms. We must avoid over-chlorination, which can lead to the formation of harmful disinfection byproducts.
The pulp and paper industry also uses this element in the bleaching of wood pulp. Accurate knowledge of its molar mass, therefore, is vital to control the bleaching process, optimizing product quality and minimizing environmental impacts.

Natural disinfectants like aquarium salt can kill dangerous bacteria and parasites that enter the aquarium.
©Vojce/Shutterstock.com
6. Pharmaceutical Industry:
The pharmaceutical industry also heavily relies on chlorine-containing compounds due to their usefulness in drug development and manufacturing. Knowing the molar mass of this element is essential during drug formulation. This is because precise dosing ensures the consistency and efficacy of pharmaceutical products. It also aids in quality control, ensuring that the final drug product contains the correct amount of chlorine-containing compounds.
Real-World Examples of Chlorine Uses
As we know, chlorine is a versatile element with a wide range of real-world applications, owing to its unique chemical properties. Let’s explore some of the most prominent uses and examples of this element in various fields:
1. Water Treatment:
Municipal water treatment plants extensively use chlorine to disinfect drinking water and wastewater.
It effectively kills or inactivates harmful microorganisms, such as bacteria and viruses, making water safe for human consumption. It also helps control algae and remove unwanted odors and tastes from water sources.
Chlorine gas (Cl₂) or sodium hypochlorite (NaClO) mix in water in carefully measured amounts to achieve the desired disinfection level. The molar mass of this element is crucial here to ensure precise dosing, preventing both under-chlorination, which may lead to waterborne diseases, and over-chlorination, which can produce harmful disinfection byproducts.
2. Disinfectants and Bleaches:
Household bleach, often in the form of sodium hypochlorite, contains chlorine as a disinfecting and bleaching agent.
Chlorine-based disinfectants help sanitize surfaces, clean drinking water containers and sterilize medical equipment. Chlorine bleach is also a powerful stain remover and whitening agent for laundry.
Chlorine-based compounds release chlorine ions (Cl⁻) when dissolved in water. These ions, therefore, react with organic matter and microorganisms, breaking down their cellular structures and rendering them harmless.

Bleach containing chlorine often has free ions that clean and sterilize things.
©Jim Gimpel/Shutterstock.com
3. Pharmaceuticals:
Chlorine compounds are useful in pharmaceutical manufacturing for various purposes. These include disinfection and synthesis of active pharmaceutical ingredients (APIs).
The chemistry of this element is therefore crucial in producing medications, from antibiotics to antiseptics. Chlorine-containing compounds can also meet specific drug formulations and therapeutic requirements.
The molar mass of this element is therefore essential in pharmaceutical development, ensuring accurate dosing and maintaining consistent product quality.
4. PVC (Polyvinyl Chloride) Production:
Chlorine is also a key component in the manufacturing of PVC, a versatile plastic polymer used in pipes, cables, clothing, and more.
PVC is known for its durability, versatility, and low cost. The addition of chlorine enhances its flame resistance and chemical resistance, making it suitable for a wide range of applications.
Chlorine gas, combined with ethylene, also produces ethylene dichloride (EDC), which is a precursor to PVC. The molar mass of chlorine is therefore crucial in controlling the polymerization process and determining the properties of the resulting PVC.
5. Disinfection in Swimming Pools:
Chlorine compounds, often in the form of calcium hypochlorite or trichloroisocyanuric acid, are used to disinfect swimming pool water.
This is because chlorine-based pool disinfectants help prevent the growth of harmful bacteria and algae, ensuring safe swimming conditions.
When added to pool water, these chlorine compounds release chlorine ions, which oxidize and destroy contaminants, maintaining water clarity and hygiene.

Swimming pools are generally chlorinated to make sure bacteria and algae don’t grow inside them and affect the health of those swimming in there.
©hxdbzxy/Shutterstock.com
6. Chemical Synthesis:
Chlorine is used in various chemical syntheses, including the production of pesticides, pharmaceuticals, and synthetic rubber.
Chlorine-containing compounds also serve as crucial building blocks in the synthesis of countless chemical products. For instance, chlorination reactions introduce chlorine atoms into organic molecules, altering their properties and reactivity.
Understanding the molar mass of chlorine is therefore vital in controlling the stoichiometry of chemical reactions, because it helps chemists to design and produce specific compounds efficiently.
7. Sanitization in Food Processing:
Chlorine-based sanitizers are used in food processing facilities to disinfect equipment, utensils, and food-contact surfaces.
Chlorine-based sanitizers also help prevent foodborne illnesses by effectively killing bacteria, viruses, and other pathogens.
These sanitizers release chlorine ions, which disrupt the cellular structures of microorganisms, rendering them harmless.
8. Production of Solvents and Chemicals:
Chlorine is also used in the production of various chemicals, including solvents like chloroform and industrial chemicals like sodium hydroxide (caustic soda).
It is a versatile precursor in the chemical industry, serving as a starting material for the synthesis of numerous compounds.
Chlorine is also involved in chemical reactions that lead to the production of a wide range of products, from solvents and plastics to cleaning agents and detergents.

Hydrochloric acid is highly corrosive. Always handle it with caution.
©Kittisak Kaewchalun/iStock via Getty Images
Other Elements With a Similar Molar Mass
To gain a better perspective on chlorine’s molar mass, let’s compare it to the molar masses of other elements. Below is a table that lists some elements with molar masses close to that of chlorine:
| Element | Symbol | Molar Mass (g/mol) |
|---|---|---|
| Chlorine | Cl | 35.453 |
| Sulfur | S | 32.06 |
| Phosphorus | P | 30.97 |
| Potassium | K | 39.10 |
| Argon | Ar | 39.95 |
In the world of chemistry, the molar mass of an element or compound is a fundamental concept. The molar mass of chlorine affects countless chemical processes, from formulating compounds and conducting reactions to ensuring safe water supplies and developing pharmaceuticals. In the case of chlorine, its molar mass of approximately 35.453 g/mol plays a crucial role in all these applications.
Therefore, understanding chlorine’s molar mass allows chemists and researchers to make precise measurements, optimize chemical reactions, and maintain safety standards.
Thank you for reading! Have some feedback for us? Contact the AZ Animals editorial team.








