Ethyl alcohol, or ethanol, has journeyed with humanity from the times of ancient civilizations to our modern world. The compound was once a part of our ancestors’ fermented drinks, and now it’s used in much more than beverages. Today, ethanol is a critical part of green biofuels, and it’s a life-saving disinfectant that stops diseases from spreading. Ready to learn more? Follow along to learn the molar mass of ethyl alcohol (ethanol), plus key examples of this compound.
Molar Mass of Ethyl Alcohol
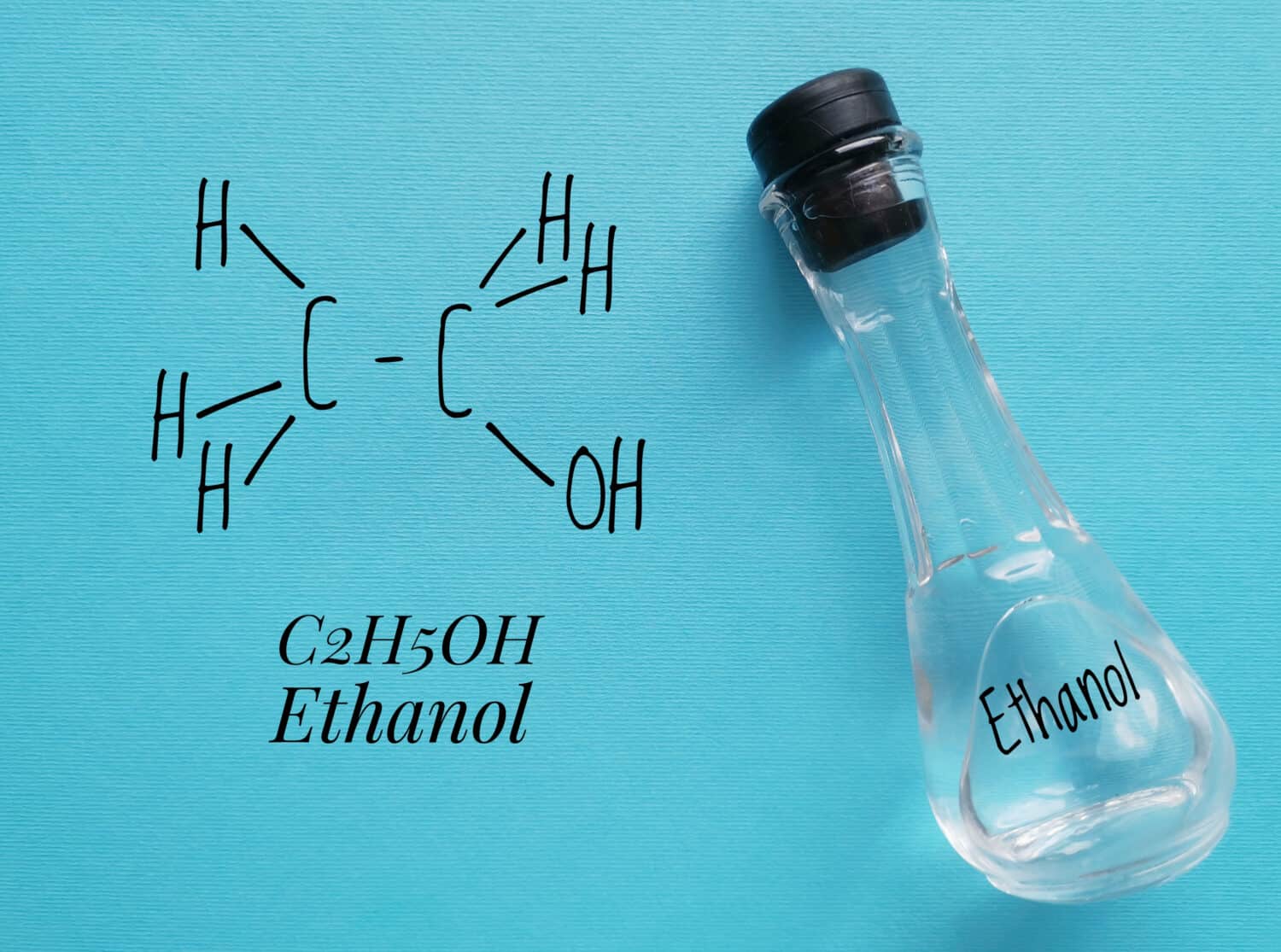
The molar mass of ethyl alcohol is about 46.08 grams per mole.
©Danijela Maksimovic/Shutterstock.com
The molar mass of ethyl alcohol is about 46.068 grams per mole. Molar mass helps us understand substances in a numerical way. This weight represents the mass of one mole of the substance, measured in grams. Moles are units that chemists use to count and compare tiny particles like atoms.
Now, why is molar mass important? Without molar mass, it’d be difficult to study and work with different elements and compounds. This measurement allows us to grasp the heaviness of one mole of a substance, which you’ll soon find out is a huge amount.
Imagine that atoms are like colorful tiny blocks. Now, think of molar mass as the weight of a bag filled with millions of these blocks. The bag contains a lot of blocks, just like one mole of a substance contains an enormous number of atoms or molecules. By knowing the entire weight of the bag, we can understand how heavy one mole of the substance is. Counting the number of blocks in the bag helps us determine how many there are. Similarly, molar mass gives us a clear picture of how much substance we are dealing with.
Calculating Molar Mass

Knowing the molar mass of carbon, hydrogen, and oxygen lets you calculate the molar mass of ethanol.
©Cherries/Shutterstock.com
How do they calculate the molar mass of ethyl alcohol? Ethanol is a compound, so we have to consider all of the elements within it. Knowing the molar mass of carbon, hydrogen, and oxygen makes it possible to calculate the molar mass of ethanol. We have to consider the atoms of carbon (C), hydrogen (H), and oxygen (O) that come together to create ethanol, represented as C2H5OH.
First, we begin with two carbon atoms, each possessing a molar mass of 12.01 g/mol. Next, we have six hydrogen atoms, each with a molar mass of 1.008 g/mol. Finally, we have one oxygen atom, which has a molar mass of 16.00 g/mol.
To find the molar mass of ethanol, multiply the atomic mass of carbon (12.01 g/mol) by 2, the atomic mass of hydrogen (1.008 g/mol) by 6, and add the atomic mass of oxygen (16.00 g/mol). This gives you a molar mass of 46.068 g/mol.
Here’s what the calculation looks like:
(2 * 12.01 g/mol) + (6 * 1.008 g/mol) + (16.00 g/mol) = 46.068 g/mol
And there you have it. The molar mass of ethanol is 46.068 g/mol.
Avogadro’s Number
As you learn about molar mass, you’ll come across Avogadro’s number. The value of Avogadro’s number is approximately 6.022 x 10^23 particles per mole. This number helps us relate the mass of a substance to the number of its particles.
If you have a certain number of moles of ethyl alcohol, you can multiply that number by Avogadro’s number (6.022 x 10^23) to calculate the approximate number of ethyl alcohol molecules. For instance, 1 mole of ethyl alcohol molecules would consist of 6.022 x 10^23 ethyl alcohol molecules. If we have two moles of ethyl alcohol, we know that we would have approximately 2 x 6.022 x 10^23 molecules of ethanol. For 3 moles of ethyl alcohol, we’d have approximately 3 x 6.022 x 10^23 molecules of ethanol.
Ethanol in Nature

Ripe fruits and decaying plants can contain ethanol.
©iStock.com/Armando Leonardo Silva
Unlike sodium, which is abundant throughout the environment, ethanol is different. Sodium can be found in large quantities in the ocean or salt caves. But the majority of the ethanol is human-made. People ferment sugars derived from crops such as corn, sugarcane, or grains to produce ethanol. It’s also manufactured in factories.
That’s not to say there isn’t any naturally occurring ethanol. It’s just not enough to have a big impact. In nature, you might find a little bit of ethanol from things like ripe fruits or decaying plants.
Real World Examples: Practical Applications

Beer, wine, and spirits all contain ethanol.
©Syda Productions/Shutterstock.com
Ethanol serves a multitude of purposes. It is used as a solvent for dissolving substances, added to fuel, and plays a role in the production of various everyday items, including alcoholic beverages and antiseptics.
Alcoholic Beverages
Beer, wine, and liquor; all contain ethanol. Knowing the molar mass of ethanol plays a significant role in the creation process, helping brewers determine the final alcohol content. And humans have been using ethanol in boozy beverages for thousands of years.
The earliest alcoholic beverage ever discovered was a mix of fermented rice, honey, hawthorn fruit, and possibly grape, containing ethanol. Found in pottery jars dating back to 7000-6600 BCE in China, this beverage predates the earliest evidence of grape wine from the Middle East by over 500 years. This finding sheds light on the ancient production of ethanol and its cultural significance, revealing advanced brewing techniques and flavoring practices. Research also suggests that these beverages played a role in burial rituals.
Antiseptic
Ethanol also exhibits strong antiseptic and disinfectant properties. This makes it invaluable in the medical and pharmaceutical fields. This powerful compound can eradicate bacteria and other harmful microbes.
Biofuel
Because of its combustible nature, ethanol is frequently utilized as a biofuel. Take, for instance, the environmentally friendly E85 or flex fuel. E85 contains 51% to 83% ethanol, depending on geography and season. This blend qualifies as an alternative fuel under EPAct. It’s primarily intended for use in flexible fuel vehicles (FFVs), which are equipped with internal combustion engines designed to run on E85.
More Fuels Using Ethanol
E85 is just the start. There’s a lot of other fuels that use ethanol, and in different ways. For instance, E10 is a fuel blend that includes 10% ethanol and 90% gasoline. It’s used in most conventional gasoline-fueled vehicles and is available in every U.S. state. The use of E10 is supported by the U.S. Environmental Protection Agency (EPA) and was prompted by the need to reduce carbon monoxide levels in the atmosphere.
E15, on the other hand, contains between 10.5% and 15% ethanol and is suitable for vehicles made in 2001 or later. There are certain regulations in place to ensure that E15 is not used in older vehicles, helping to mitigate potential issues. The Energy Policy Act of 1992 (EPAct), doesn’t recognize E15 as an alternative fuel yet. However, it contributes to fulfilling the Renewable Fuel Standard.
There are also ongoing efforts to explore the potential of high-octane fuel, particularly blends with 25% to 40% ethanol. High octane fuel is being studied to see if it can help reduce energy use and harmful gases that contribute to climate change. Researchers are looking into whether it could be a good option for the market.
Other Elements With a Similar Molar Mass
| Compound/Element | Molar Mass (g/mol) |
|---|---|
| Sodium | 22.99 |
| Phosphorous | 30.97 |
| Sulfur | 32.07 |
| Potassium | 39.10 |
| Nitrogen | 28.02 |
Looking at the table above, you’ll notice that the numbers for molar masses aren’t exactly the same as ethanol’s molar mass of 46.068 g/mol. Some, like sodium and nitrogen, have lower molar masses with values of 22.99 g/mol and 28.02 g/mol, respectively.
You might wonder why include them if they’re not that close? Well, when it comes to molar mass, it’s helpful to group things into ranges. These values may don’ match exactly, but they fall within a reasonable range to be considered similar.
So, even though sodium and nitrogen have lower molar masses compared to ethanol, they’re still in the same ballpark. They’re part of the similar measurement class because their molar masses are relatively close to ethanol’s, even if not identical.
Final Thoughts on the Molar Mass of Ethyl Alcohol
The molar mass of ethyl alcohol is approximately 46.068 grams per mole. And that’s a number that represents a whole lot of molecules packed into a small space. Calculating the molar mass of ethanol involves considering the atomic masses of carbon, hydrogen, and oxygen, the elements that compose it. Ethanol consists of two carbon atoms, six hydrogen atoms, and one oxygen atom, forming the chemical formula C2H6O.
We also learned that ethanol has had a major impact on human history. The journey starts back with ancient boozy drinks and follows into today where ethanol helps with all sorts of modern stuff. It’s even being explored as an alternative fuel source. Most of the time, humans make ethanol by fermenting sugars from crops like corn, sugarcane, or grains. But believe it or not, it can also be found naturally in small amounts in ripe fruits or decaying plants. Still, the majority of it is made in factories.
Keep the momentum going! This article is just the beginning of your journey to understanding molar mass. To truly grasp the concept, dive deeper into how it applies to other elements and compounds. Check out molar mass calculations for sodium chloride or glucose, for example. The more you explore, the better you’ll understand this fundamental concept.
Thank you for reading! Have some feedback for us? Contact the AZ Animals editorial team.








