Nitrogen makes up a whopping 78 percent of Earth’s atmosphere. Nestled between carbon and oxygen on the periodic table, nitrogen is represented by the symbol, N. Nitrogen atoms seldom travel alone. They form incredibly strong bonds with other nitrogen atoms to form N2 molecules, or with other elements to form a variety of familiar compounds such as ammonia NH3, nitrous oxide N2O, and nitric acid HNO3. The molar mass of nitrogen is an important property of this element.
Nitrogenous compounds are necessary for life. Nitrogen is in our foods, our water, and even in our DNA. It is essential for everything from fertilizing crops and putting whipped cream on our desserts to blowing up bridges. At standard temperature and pressure, nitrogen exists as a gas. It has no color and no odor. It has a low, low boiling point of −195.8 °C (−320.4 °F), below which it is naturally a liquid at normal atmospheric pressure.

Liquid nitrogen turns to vapor very quickly at room temperature.
©zkifuk/iStock via Getty Images
This article will explore the molar mass of nitrogen, what that means, and why it is important. Every element has a defined molar mass. So, too, does every compound. The molar mass of a substance influences its physical and chemical properties. A substance’s molar mass is simply the mass, in grams, of one mole of that substance.
In chemistry, a mole is a set number of particles, whether atoms or molecules. That number is a constant, meaning it is the same, every time, no matter what. The number of particles in a mole is approximately 6.022 × 1023 units, also known as Avogadro’s number or Avogadro’s constant. Named for the Italian scientist, Amadeo Avogadro, this constant is extremely important in the understanding of chemistry and how the world works.
The Molar Mass of Nitrogen
The molar mass of nitrogen is 14.0067 grams per mole. That means one mole of nitrogen atoms, or 6.022 × 1023 nitrogen atoms, has a mass of approximately 14 grams. For comparison, one mole of hydrogen atoms has a molar mass of approximately one gram. Let’s take a look at these two elements and how their molar masses are determined.
Hydrogen is the first element on the periodic table. It has only one proton per atom, and no neutrons. The mass of hydrogen’s one miniscule electron is negligible, and the single proton has a mass of approximately one atomic mass unit. One mole of hydrogen atoms has a mass of 1.00784 grams per mole, which for our purposes can be rounded to 1 gram per mole.
Nitrogen is the seventh element on the periodic table. It has an atomic number of seven, with exactly seven protons. Almost all nitrogen atoms in nature also have seven neutrons. Both protons and neutrons have the same approximate mass, and again, the electrons in these atoms have a negligible mass. Therefore, as expected, the molar mass of nitrogen is approximately the total of seven moles of protons plus seven moles of neutrons, or 14 grams. In fact, the calculated mass of one mole of nitrogen atoms is 14.0067 grams.
Isotopes Make a Difference
One might wonder why the molar mass of nitrogen is not exactly 14 grams, but instead is just slightly larger. The difference lies in the distribution of isotopes of the element found in nature. Most elements on the periodic table exist in more than one known form. These forms, called isotopes, have the same number of protons, but a different number of neutrons. Usually, a primary isotope accounts for the majority of the atoms that exist naturally, while one or more other isotopes make up the rest. For nitrogen, the main isotope is 14N, which has seven protons and seven neutrons in its nucleus. The other isotope, 15N, has seven protons and eight neutrons.
To calculate the actual molar mass of an element, one must take into account all the different naturally occurring isotopes of that element. To discover the molar mass of nitrogen or any other element with more than one naturally occurring isotope, scientists average the atomic masses of the known isotopes. They calculate the percentage of each isotope as found in nature, and express the average in grams. In other words, multiply the percentage of each isotope by its individual molar mass, and then add the totals for each isotope together to find the average molar mass.
Frequency of Each Isotope
Nitrogen has two main naturally occurring isotopes, but they do not appear with the same frequency. The 14N isotope occurs much more frequently than the 15N isotope, with a ratio of 99.634 percent 14N atoms and 0.366 percent 15N atoms. Scientists determine the atomic mass of an element by averaging the masses of all the different naturally occurring isotopes with respect to the frequency with which they are actually found. Since a small percentage of nitrogen atoms exist as the 15N isotope, the average molar mass is slightly larger than 14 grams per mole.
Elemental Nitrogen in Nature
Elemental nitrogen exists as a gas in its natural state. It is one of a handful of elements, including hydrogen, oxygen, fluorine, chlorine, bromine, and iodine, which form diatomic molecules. That means, rather than existing as single atoms, two atoms of these elements join together to form strongly bonded molecules. For example, the oxygen we breathe exists primarily as an O2 molecule, rather than single oxygen atoms in the atmosphere. So, too, elemental nitrogen most often exists as the N2 gas.
Atomic Structure of Nitrogen
Nitrogen, with an atomic number of seven, is in the second period of the periodic table, in group 15. As previously mentioned, nitrogen has seven protons in its nucleus, along with, usually, seven neutrons. Nitrogen also has seven electrons. The arrangement of an atom’s electrons affects its behavior and how it bonds with other elements. Like other elements in the second period, nitrogen has two electrons in its inner shell, and a specific number of electrons corresponding with its group in its outer shell. For nitrogen, this number is five.
When forming chemical bonds, atoms generally work toward achieving the most stable configuration they can, whether by giving or receiving electrons through ionic bonding, or by sharing them via covalent bonding. The most stable electronic configuration for an element is generally that of the nearest noble gas, or member of group 18 (formerly listed as group VIIIA in the United States). For nitrogen, the nearest noble gas is neon, with eight electrons in its fully filled outer shell. That means each nitrogen atom, with five electrons in its outer shell, will form bonds to achieve that highly stable noble gas configuration.
Strong and Stable Nitrogen Bonds
Elemental nitrogen, as mentioned above, exists in nature primarily as a diatomic molecule, N2. But how do both of the nitrogen atoms in this pair get to the desired noble gas configuration if each one only has five electrons in its outer shell? The answer lies in covalent bonds.
Covalent bonds allow atoms to share electrons. Each nitrogen atom in an N2 molecule shares three of the electrons in its outer shell with the other nitrogen atom. They form a triple bond, one of the strongest bonds. The three pairs of shared electrons move at high speed in a cloud around both nuclei, giving each nitrogen atom in the molecule the desired eight electrons it needs to achieve a stable configuration.
Other Familiar Compounds
Nitrogen forms many familiar compounds, bonding readily with other elements. As shown below, nitrogen and oxygen combine to form multiple compounds. Nitrogen dioxide, NO2, is a pollutant that forms when fossil fuels are burned. You might have inhaled N2O, also known as nitrous oxide, before undergoing a dental procedure or used it to propel whipped cream onto your favorite dessert. Meanwhile, nitric oxide, NO, is essential for circulatory regulation and a healthy immune system.
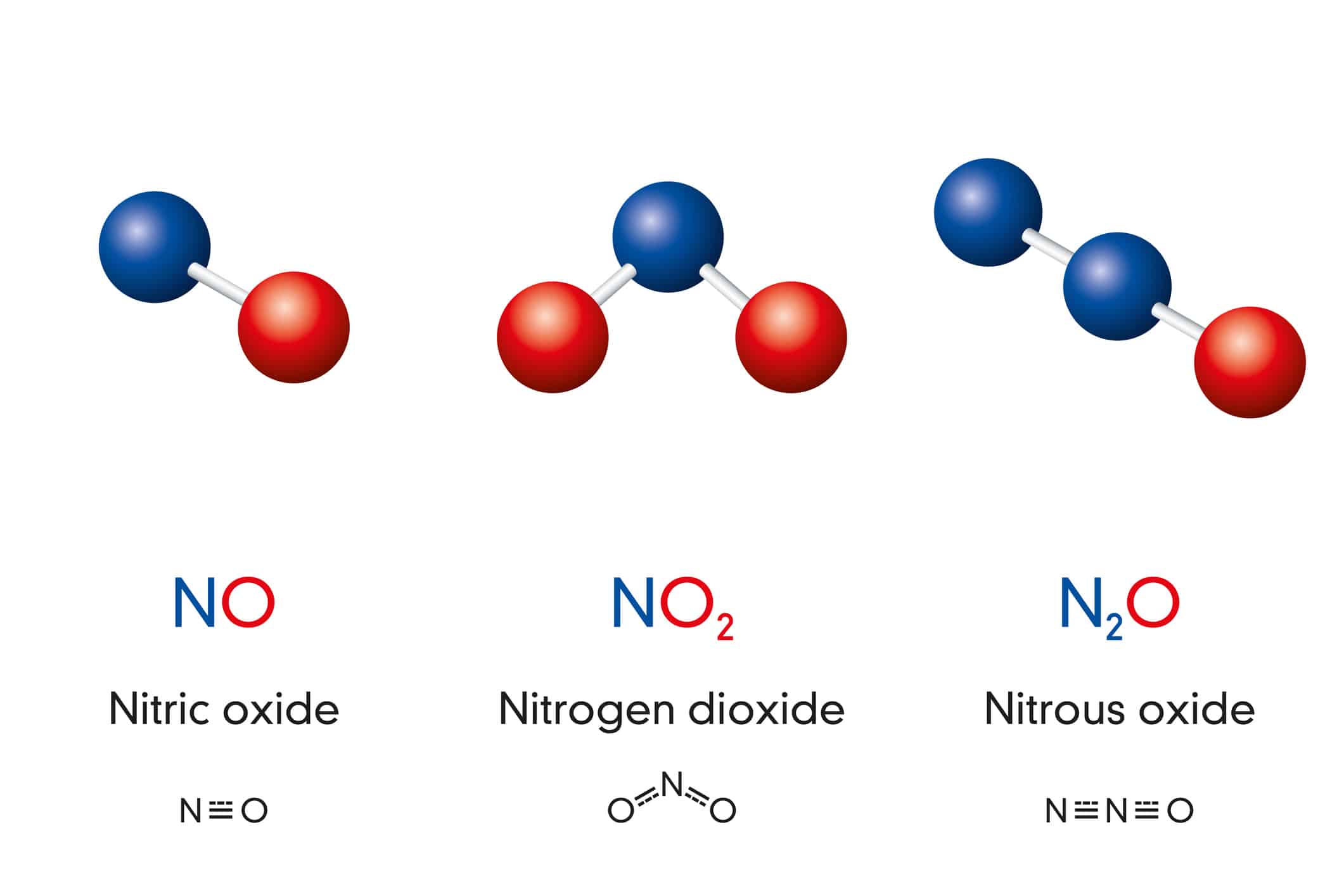
Nitrogen and oxygen can combine to form multiple compounds.
©PeterHermesFurian/iStock via Getty Images
If you have any sense of smell, you can probably recognize one particular nitrogenous compound easily. When one nitrogen atom bonds with three hydrogen atoms, it forms NH3, also known as ammonia. Remember that each nitrogen atom needs three extra electrons to reach the noble gas configuration it desires. Each hydrogen atom has one single electron it can share. Therefore, NH3 is a very stable compound.
When a nitrogen atom bonds with four hydrogen atoms, it forms the NH4+ ammonium ion. This polyatomic ion is essential in forming a large variety of ammonium compounds. NH4ClO4, ammonium perchlorate, can be used as a rocket propellant. Ammonium nitrate, NH4NO3, is a highly effective fertilizer used in agriculture. But it can also be used to create powerful explosives such as the bomb that destroyed the Alfred P. Murrah Federal Building in Oklahoma City in 1995, killing 168 people. Fertilizer bombs such as these are horrific, but much less powerful than the atomic bomb designed by J. Robert Oppenheimer, or the even more destructive hydrogen bomb, which Oppenheimer opposed.

Ammonium nitrate can fertilize crops, but it can also be used to make deadly bombs.
©Kittisak Kaewchalun/iStock via Getty Images
The Molar Mass of Nitrogen Gas
As we already learned, the mass of one mole of nitrogen atoms is approximately 14.0067 grams. But nitrogen gas has not just one atom of nitrogen, but two atoms bonded tightly together in each molecule. So, one mole of nitrogen molecules actually equals two moles of nitrogen atoms. Therefore, to find the molar mass of nitrogen gas, with the formula N2, one would simply multiply the molar mass of the singular elemental nitrogen by two. The molar mass of nitrogen gas is approximately 28.0134 grams per mole.
Other Elements with a Similar Molar Mass
The periodic table is arranged with each element in order based on its atomic number. Remember, the atomic number is the number of protons in each atom of the element. Nitrogen has seven protons in each atom, giving it the atomic number seven. Its molar mass is just over 14 grams per mole. The closest two neighbors of nitrogen are carbon, with an atomic number of six and a molar mass of approximately 12 grams per mole, and oxygen, with an atomic number of eight and a molar mass of roughly 16 grams per mole.
The chart below illustrates the relationships between the atomic number and the molar masses of the elements in the second period of the periodic table from boron through neon. These elements each have two electrons in both the first and second S-orbitals, and between one and six electrons in the first set of P-orbitals.
| Element | Atomic Number | Molar Mass | State at Standard Temp. and Pressure |
|---|---|---|---|
| Boron | 5 | 10.81 g/mol | Solid |
| Carbon | 6 | 12.011 g/mol | Solid |
| Nitrogen | 7 | 14.007 g/mol | Gas (Diatomic Molecule) |
| Oxygen | 8 | 15.999 g/mol | Gas (Diatomic Molecule) |
| Fluorine | 9 | 18.998 g/mol | Gas (Diatomic Molecule) |
| Neon | 10 | 20.180 g/mol | Gas (Inert) |
Conclusion
Nitrogen is literally all around us. As the most abundant element in the atmosphere, nitrogen is everywhere we look and in every breath we take. However, obtaining the element in a usable form requires a complex process called the nitrogen cycle. Plants and microbes pull nitrogen from the air and convert it to usable compounds in the soil. Nitrogen cycles through plants, fungi, and other microbes. Then other organisms, including humans, consume the fixed nitrogen and use it to build our own important compounds, including nucleic acids needed for DNA and RNA, and amino acids to grow our muscles. Eventually, through decomposition and more microbial activity, nitrogen gas is released into the atmosphere to begin the cycle all over again.
On an atomic level, nitrogen contains just seven tiny protons and seven neutrons. It is the seventh lightest element known to exist. Only six others have a smaller molar mass. But nitrogen is undoubtedly one of the most important elements in our world.
The photo featured at the top of this post is © zkifuk/iStock via Getty Images
Thank you for reading! Have some feedback for us? Contact the AZ Animals editorial team.







