Life is a complex tapestry of interwoven processes, many of which occur at microscopic scales yet have profound implications for the bigger picture. Among these are the fascinating phenomena of hypertonic vs. hypotonic solutions. We will explore understanding the effect of solutes on cells as the primary objective. While they might seem like lofty scientific concepts reserved for the pages of a biology textbook, they are more straightforward than one may think, and they permeate our everyday lives in ways we might not even realize.
In this article, we will delve into the world of hypertonic and hypotonic solutions, exploring their fundamental principles, their pivotal roles in cellular processes, and their practical applications in medical and industrial contexts. Prepare to uncover the extraordinary within the ordinary.
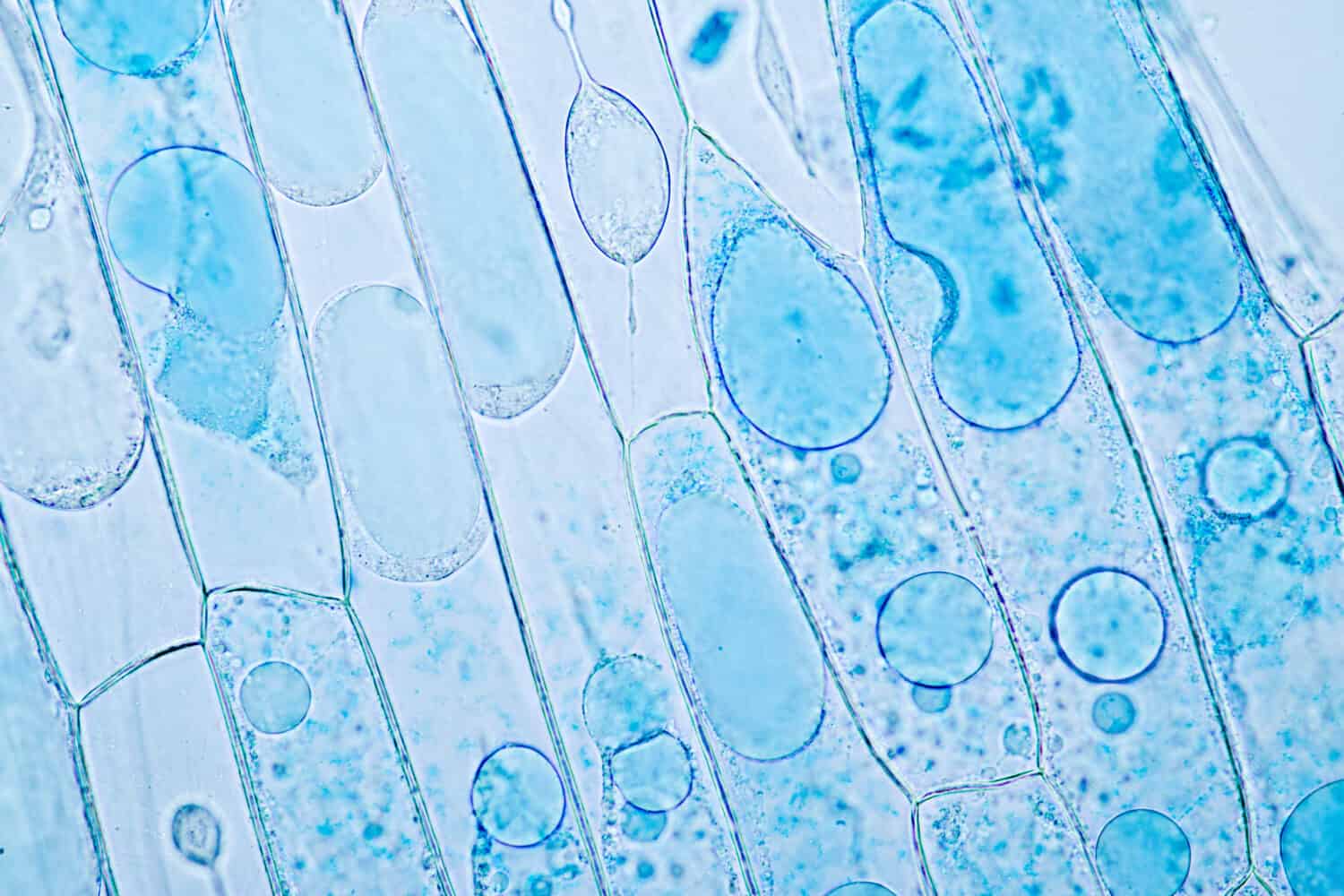
In hypertonic, ‘hyper’ stands for ‘over’ or ‘more,’ and ‘tonic’ pertains to tension. In hypotonic, ‘hypo’ stands for ‘under’ or ‘less.’
©Rattiya Thongdumhyu/Shutterstock.com
Definitions and Concepts of Hypertonic and Hypotonic Solutions
Let’s take a fundamental approach and view these solutions from a purely chemical perspective.
Even though these terms have roots in biology, they’re fundamentally about the fascinating dance of concentration gradients in chemical solutions.
Hypertonic
In hypertonic, ‘hyper’ stands for ‘over’ or ‘more,’ and ‘tonic’ pertains to tension. A hypertonic solution, like the mighty elephant in the room, has a ‘bigger’ or ‘higher’ concentration of solutes when compared with another solution. Picture two glasses of lemonade on a hot summer day. One glass is made with one lemon and a cup of water, while the other is made with two lemons and the same amount of water. The second glass would be hypertonic to the first because it has a higher concentration of lemon juice, our solute in this case.
Hypotonic
Conversely, in a hypotonic, ‘hypo’ means ‘under’ or ‘less.’ So, a hypotonic solution, like a tiny hummingbird, has a ‘smaller’ or ‘lower’ concentration of solutes when compared with another solution. Consider the same two glasses of lemonade. If a third glass of lemonade is made with half a lemon and a cup of water, it would be hypotonic to both the first and second glasses, as it has a lower lemon juice concentration.
Comparing Both Solutions
The comparisons of ‘elephant‘ and ‘hummingbird‘ aren’t by chance; they illustrate the relative nature of these terms. A solution isn’t inherently hypertonic or hypotonic. Only when you compare two solutions do these labels come into play. The strength of a lion may be significant on land, but in the ocean, it doesn’t compare to the might of a whale. So, a solution can switch from being hypertonic to hypotonic when compared with different solutions.
Now you may wonder, why bother about such concepts? Understanding these basic chemical relationships is vital to unlocking more complex processes, not just in biology but also in environmental science, food chemistry, and more. While these applications may venture into the realms of osmosis, cellular processes, or industrial uses, the underlying principles of hypertonic and hypotonic solutions are rooted in these fundamental understandings.

Hypertonic and hypotonic chemical relationships are not just found in biology but also environmental science, food chemistry, and more.
©Stock-Asso/Shutterstock.com
Differences Between Hypertonic and Hypotonic Solutions in Terms of Osmolarity and Water Movement
The word ‘balance’ often takes center stage in the natural world. Be it the delicate equilibrium of an ecosystem or the intricate dance of the changing seasons, balance is an ever-present principle. On a much smaller scale, right down at the molecular level, a similar story of balance unfolds, with osmolarity and water movement in solutions. Let’s delve further into the differences between hypertonic vs. hypotonic solutions relating to osmolarity and water movement.
Let’s Paint a Picture
Picture a bustling pond full of energetic ducks. The pond, teeming with life, can be compared to a hypertonic solution with a higher osmolarity than another solution. Osmolarity, akin to the number of ducks in our pond, is a measure of solute concentration, defined as the number of solute particles per liter of solution.
In a hypertonic solution, there’s a veritable ‘duck party’ going on, where more solute particles are present per unit volume. But nature is all about balance, remember? So, what does this overcrowded pond prompt? Just as some ducks might prefer to swim off to a quieter part of the lake, water molecules, too, prefer to move from the area of lower solute concentration to that of higher concentration.
Think about the difference between a spoon of sugar in a cup of tea versus the same spoon of sugar in a swimming pool. The water molecules in the tea will be more likely to interact with sugar particles than those in the pool, where sugar particles are few and far between. This movement of water molecules from lower to higher concentrations of solutes is the crux of the concept of hypertonic solutions.
Now, imagine a serene and quiet corner of the lake, a haven away from the bustling duck party. This tranquil setting mirrors a hypotonic solution, where osmolarity is lower than another solution. In other words, it’s a less crowded duck gathering, so there are fewer solute particles present per unit volume.
And how does the water respond to this peaceful setting? Just as more ducks might be drawn to the tranquility of the quiet corner, water molecules also flow towards the area of lower solute concentration. This occurrence is water’s way of restoring balance and striving to create an even distribution of solute particles.
Takeaways
An interesting takeaway from these principles is how the terms ‘hypertonic’ and ‘hypotonic’ are comparative. A solution isn’t inherently hypertonic or hypotonic. Once again, only when you compare two solutions will these labels come into play.
Understanding these fundamental principles of hypertonic and hypotonic solutions, osmolarity, and water movement opens up a fascinating world of insight. You might wonder, ‘How does this connect to real life?’ Well, consider the act of brewing your morning coffee or making lemonade on a hot summer day. When looking for that perfect flavor, you’re intuitively trying to strike a balance between creating a solution that isn’t too hypertonic (too strong) or too hypotonic (too weak).
From brewing beverages to understanding the properties of seawater compared to freshwater, the principles of osmolarity, hypertonicity, and hypotonicity are all at play. Without even realizing it, we’re all chemists in our own right, skillfully manipulating the concentrations of various solutions in our daily lives.
The concepts of hypertonic and hypotonic solutions, characterized by differences in osmolarity and the resulting movement of water, underscore the principle of balance fundamental to natural phenomena. Let’s remember these subtle dances happening in every solution around us, each playing its part in the grand landscape of life.

A solution isn’t inherently hypertonic or hypotonic. It is only when you compare two solutions will these labels come into play.
©ganjalex/Shutterstock.com
Biological Significance of Hypertonic and Hypotonic Solutions in Cellular Processes
Each organism thrives by maintaining a delicate internal balance, whether a towering elephant or a microscopic bacterium. As we shift our focus to the cellular processes, we will uncover a fascinating interplay between hypertonic vs. hypotonic solutions shaping life on the microscopic level.
Osmosis plays a critical role in the cellular processes. So, it’s worth mentioning again that osmosis refers to the movement of water across a semi-permeable membrane. In the cellular processes, this would be water moving across the cell wall. From an area of lower solute concentration to an area of higher solute concentration. And what’s the end goal of this process? Once again, achieving equilibrium.
Hypertonic Solutions in Cellular Processes
A hypertonic solution has a higher concentration of solutes (like salts or sugars) outside the cell than inside it. So what does this mean for our cells? When a cell finds itself in a hypertonic solution, water flows out of the cell into the surrounding solution to even out the concentrations. This results in the cell shrinking. In extreme scenarios, the cell can undergo a process called plasmolysis, leading to potential cell death.
Imagine a saltwater fish suddenly finds itself in freshwater. The fish’s body cells adapt to a salty environment, which is hypertonic to their interior. But the freshwater is hypotonic compared to the fish’s cells. This leads to an excessive intake of water by the cells. Potentially causing them to burst, which is a process known as lysis.
Hypotonic Solutions in Cellular Processes
A hypotonic solution has a lower concentration of solutes outside the cell than inside it. This state leads to water moving into the cell to balance out the concentrations, causing the cell to swell. The cell could burst if the inflow isn’t properly controlled.
To reiterate, while the terms hypertonic and hypotonic might sound intimidating, they essentially describe the balance and movement of water in response to solute concentrations. From maintaining the health of our cells to helping fish adapt to their environments, the processes underpinning these concepts are essential to life as we know it.
Examples of Cellular Processes
You may still wonder why these solutions and the resulting water movements are of such importance. These principles are at the heart of many biological processes. This includes nutrient absorption, waste removal, and maintaining cell volume and shape, which are critical for cellular function and survival.
For instance, red blood cells exemplify this beautifully. They need to maintain their biconcave shape and volume for efficient oxygen transport. If placed in a hypertonic solution, they lose water, shrivel up, and their ability to carry oxygen is compromised. In a hypotonic solution, too much water can cause them to burst, a process called lysis, again hampering oxygen transport.
Plants demonstrate the importance of these principles. When in a hypotonic environment, their cells take up water, generating turgor pressure. This process is a plant’s version of a skeleton, which helps maintain the plant’s structure and is the reason why watering plants keeps them upright and prevents wilting.
Our bodies also deftly manipulate these principles. Kidneys adjust the hypertonicity of urine to regulate our body’s water content. When you notice your urine is darker, it is hypertonic because you are dehydrated and need to conserve water. Your urine is hypotonic when it is clearer, and you’ve had plenty of water.
From the cells in our body to plants standing tall, understanding the balance between hypertonic and hypotonic solutions is pivotal to life as we know it.
The delicate interplay of these solutions, the resulting water movement, and the balancing act of osmolarity are not just scientific concepts but keynotes in the symphony of life. It’s a reminder that even in the tiniest of cells, the principle of balance reigns supreme. Underlying the biological processes of life itself.

Hypertonic and hypotonic essentially describe the balance and movement of water in response to solute concentration.
©bogdanhoda/Shutterstock.com
Practical Applications of Hypertonic and Hypotonic Solutions in Medical and Industrial Settings
Imagine you’ve been swept up in the excitement of a marathon, running with all your might. Suddenly, you feel a pang of exhaustion. What do you reach for? Most likely, it’s a sports drink, a practical application of hypertonic solutions. The drink is formulated to be hypertonic compared to body fluids. This means it contains more solutes, usually a mix of salts and sugars. This higher concentration helps replenish the nutrients lost during exercise. It draws water into your bloodstream to combat dehydration, helping you regain energy and keep running.
Within Medical Settings
The medical world, too, makes good use of hypertonic solutions. Doctors pay close attention to the tonicity of intravenous fluids administered to patients. The use of a hypertonic saline solution would be to draw fluid out of swollen cells. In contrast, the use of a hypotonic solution is to rehydrate dehydrated cells. As such, have you ever heard of intravenous drips? These often contain hypertonic solutions like saline or dextrose. These help replenish fluids, correct electrolyte imbalances and nourish the body when a patient cannot eat or drink.
Ophthalmologists also use hypertonic solutions. A classic example is the treatment of edema or swelling in the cornea of the eye. Hypertonic saline eye drops, having a higher solute concentration than the eye fluids, draw out excess water from the swollen cornea, relieving discomfort.
Hypotonic solutions find utility in the treatment of conditions like hypernatremia. This is where the sodium level in the blood is too high. Administering a hypotonic solution can help restore these levels to normal, avoiding complications such as fluid overload.
On the other hand, hypotonic solutions, like our calm, serene pond, have lower solute concentrations than other solutions. One of their prime uses is in treating cells gently. In laboratory settings, they are often used to cause cells to swell, making them easier to study under the microscope.
Within Industrial Settings
In the industrial realm, hypotonic solutions play a significant role in water treatment. They are used to lower the osmolarity of wastewater. This process makes it safer to be released back into the environment or to be reused. A crucial part of environmental conservation efforts.
Beverage industries, too, have a stake in the hypotonic game. Many sports drinks marketed for consumption during light to moderate exercise are made hypotonic. They are designed to quickly hydrate the body and replenish lost electrolytes without providing excessive nutrients that could slow down absorption.
Our understanding of hypertonic vs. hypotonic solutions extends beyond the textbooks and impacts many aspects of our daily life. These concepts have found practical applications in medicine, industry, sports, and even in the humble act of rehydrating our bodies. Let’s take a moment to appreciate the dances of water molecules in response to hypertonic and hypotonic environments.
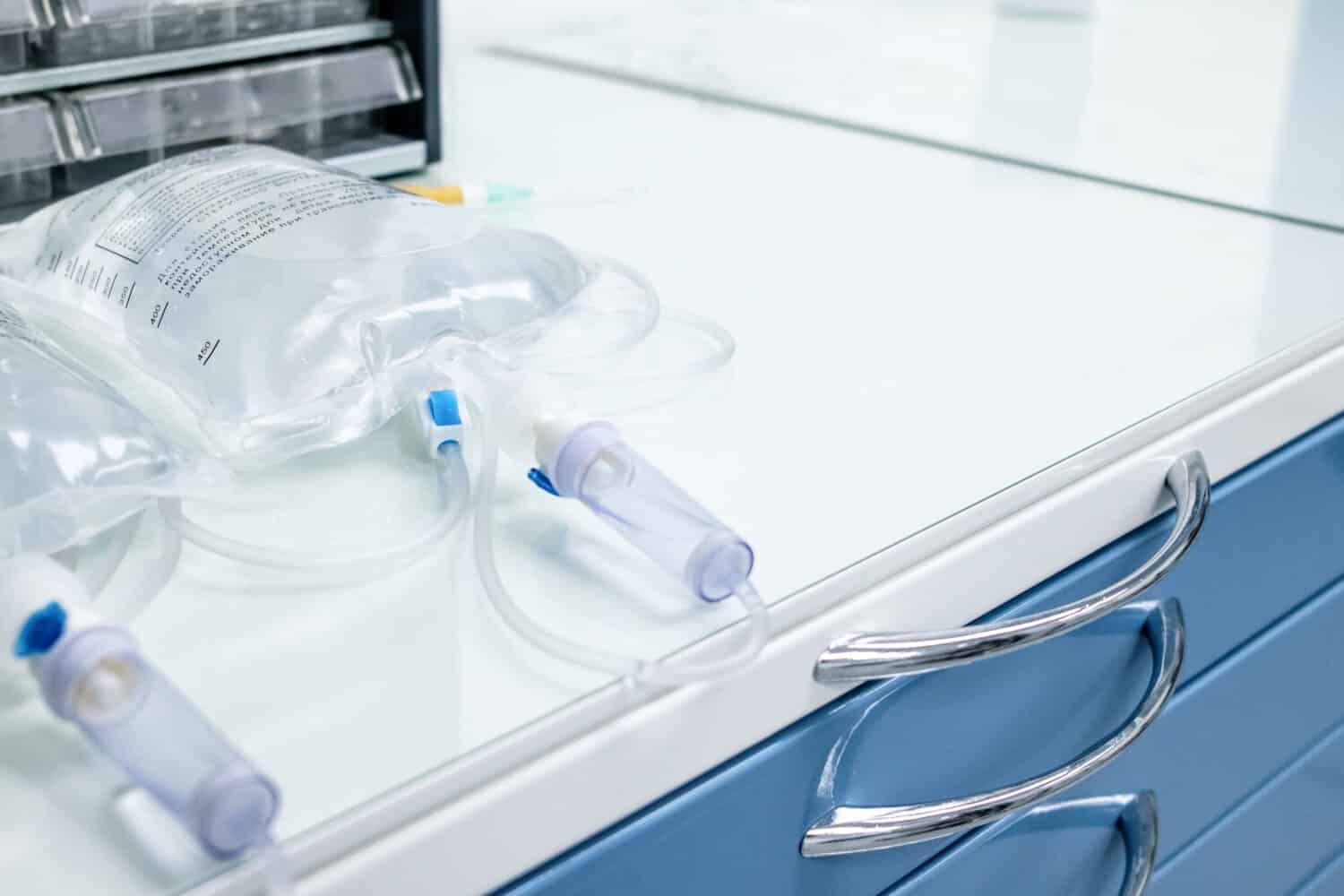
Intravenous drips often contain hypertonic solutions, like saline or dextrose.
©Nekrasov Eugene/Shutterstock.com
Conclusion
As we’ve journeyed through the fascinating world of hypertonic vs. hypotonic solutions, we’ve discovered their invaluable contributions to life. These solutions are working hard in the world around us. Influencing both the minute cellular dances and the larger arcs of biological processes. They play vital roles in nutrient absorption, waste removal, and maintaining cellular shape and volume. As well as in oxygen transport, underscoring life as we know it.
Their magic extends beyond the microscopic, finding practical applications in our daily life, particularly in medical and industrial settings. These solutions underscore our existence. In essence, the understanding and application of hypertonic and hypotonic solutions remind us of the beauty and interconnectedness of life, from the microscopic to the macroscopic. After all, every creature, every cell, and every molecule has its part in the circle of life.
Thank you for reading! Have some feedback for us? Contact the AZ Animals editorial team.







