Pretty much everyone played with magnets as kids, holding up the north side of one magnet to the south side of another and watching the ends click together. Maybe you played with magnetic balls or letters on your refrigerator.
However, magnets are a little bit more complicated than our childhood experiments with them make them seem. There are a few different types of magnets, most of which can be quantified as being either paramagnetic or diamagnetic. By understanding each of these types and their differences, you can have a deeper understanding of magnets and their properties.

This is the classic magnet we all recognize. But magnets are far more complex than just a bar with a north and south side!
©ShutterStockStudio/Shutterstock.com
Definitions
The first step to understanding the differences between paramagnetism and diamagnetism is to define what each of those is in the first place!
Here’s the context where paramagnetism and diamagnetism fit in. Consider a magnetic field, which can be defined as “the space in which a magnetic force is exerted.” The magnetic field encompasses the area outside of and within a magnetized object. If you hold a magnet that you put against the refrigerator, that magnet creates a magnetic field around it. Another way to make a magnetic field is through a wire carrying a current.
Now that you know what a magnetic field is, let’s dive into our two types of magnetism.
Paramagnetism
Paramagnetic materials are equipped with a slight positive charge. This makes the material susceptible to magnetism. Paramagnetic materials have some unpaired electrons, so those electrons essentially search for a partner in magnetic fields. When the magnetic field is gone, the electrons settle down, and the material is no longer magnetic. If you held up a paramagnetic material to a horseshoe magnet, it would exhibit magnetism and move toward the object.

A horseshoe magnet creates a magnetic field. Paramagnetic materials will be drawn into the magnet!
©ismagilov/iStock via Getty Images
Diamagnetism
Diamagnetic materials are essentially the opposite of paramagnetic materials. They are slightly negatively charged, so they reject magnetic materials in a magnetic field. All electrons in diamagnetic materials already have partners, so they aren’t searching to pair up when inside a magnetic field. If you held up a diamagnetic material to a horseshoe magnet, it would repel it.
Key Differences
In order to understand paramagnetic and diamagnetic materials, it’s important to identify the differences between the two types. The key differences are in susceptibility and behavior.
Susceptibility
In magnetism, susceptibility is the amount a material is drawn into a magnetized field. In layman’s terms, susceptibility is essentially how magnetic the object is. Paramagnetic materials are magnetic. They have a positive level of susceptibility. However, because diamagnetic materials repel magnetic materials in a magnetic field, they have a negative level of susceptibility.
One important nuance here is that paramagnetic materials are only slightly magnetic. They don’t have a very high level of susceptibility compared to their highly magnetic counterpart, ferromagnetism. But to keep it simple, just remember that paramagnetic materials have positive susceptibility, while diamagnetic materials have negative susceptibility.
Behavior

Magnets can behave in a lot of different ways.
©PCH-Vector/iStock via Getty Images
Within a magnetic field, there are weaker regions and stronger regions. When considering the behavior of paramagnetic materials and diamagnetic materials, it’s important to know the regions they are most drawn to.
Paramagnetic materials get drawn to the stronger regions of a magnetic field. This is because of their positive susceptibility. Let’s use a party as a metaphor. The party is the magnetic field, and the paramagnetic material is an extrovert. Extroverts want to be where the most people are! Likewise, paramagnetic materials want to be where the most magnetic energy is. That’s why they’re drawn to the stronger regions.
Diamagnetic materials that repel magnetism and have a negative susceptibility. When scientists analyze the behavior of diamagnetic materials, they see that these materials move to weaker regions of the magnetic field. Think, again, about the party, but this time, diamagnetic materials are the introverts. They have to be at the party, just like diamagnetic materials have to be in the magnetic fields. But introverts are going to find the rooms with the least amount of people. Likewise, diamagnetic materials are going to seek out the regions of the magnetic field that are the weakest.
Key Similarity
While paramagnetic and diamagnetic materials are opposite in many ways, there is one important similarity between the two materials. Both paramagnetic and diamagnetic materials lose their magnetic properties when they are not in a magnetic field. That means, in essence, diamagnetic materials aren’t repelling anything when they’re not in a magnetic field, and paramagnetic materials aren’t being drawn to anything. They have to be in a magnetic field for those properties to take place.
Measurement & Analysis
In the definitions of paramagnetism and diamagnetism, the concept of electron configuration was introduced. Paramagnetic materials have some unpaired electrons. They are searching for a partner, which makes them attractive in a magnetic field. In contrast, diamagnetic materials have totally paired electrons. No electrons need to go looking for partners, so they actually repel the opportunity by having negative susceptibility.
Understanding electron configuration is the key to understanding whether or not a material is paramagnetic or diamagnetic.
How can you determine the electron configuration of a material?
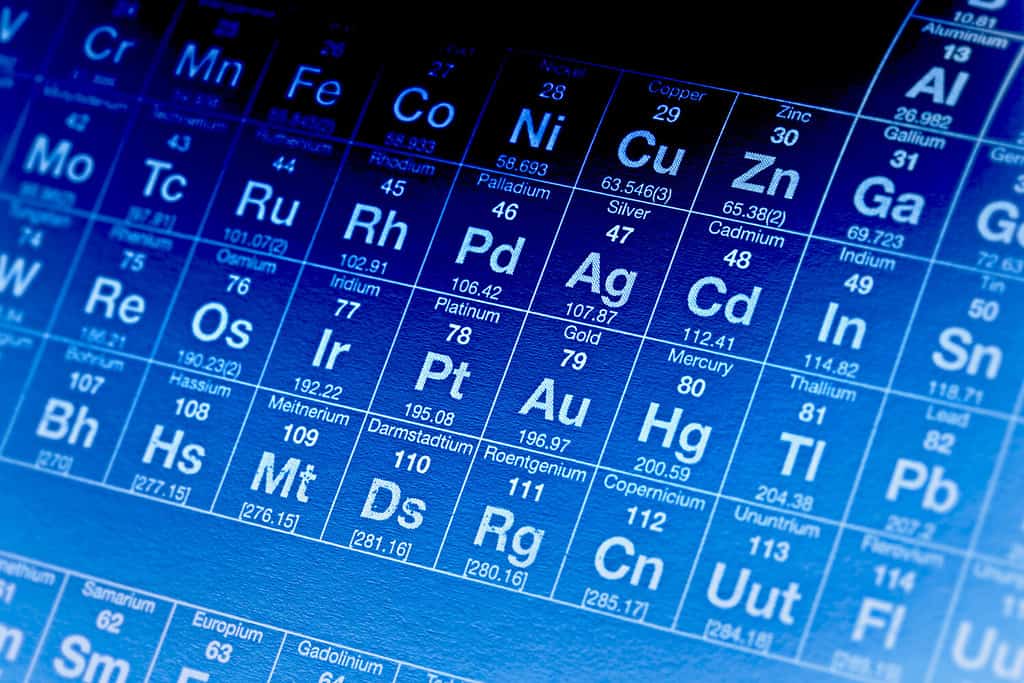
The number right above the letters and below the full name is equivalent to the number of electrons in the element.
©isak55/Shutterstock.com
The full method of determining the electron configuration of a material is a bit complicated. The root of the answer, though, is in the periodic table. The elements on the periodic table are ordered by the level of electrons they have present. The atomic number is what will tell you the number of electrons in the element.
Once you’ve identified the element of your material, you need to use shell and subshell notation to determine the exact configuration. Unless you’re a chemist or chemistry student, you probably won’t need to do this yourself. Thankfully, you can look up the electron configuration of all the elements on the periodic table, which will tell you whether or not there are loose electrons looking for a partner.
Keep in mind that there are more types of magnetism than the two introduced in this article. While electron configuration gives you the key to understanding magnetism at its basic level, there is a bit more nuance when it comes to determining the exact type of magnetism each element possesses. The elements are complex and there are a lot of factors that go into magnetism! Generally, however, elements that have unpaired electrons are paramagnetic, and elements with paired electrons are diamagnetic.
Applications
Now you know there are two primary types of magnetism! That’s all swell and dandy but… what does society use each for? Here are some applications of paramagnet and diamagnetic materials.
Paramagnetic Materials
Paramagnetic materials show up in a lot of different industries. The tech industry uses paramagnetic materials to produce sensors, and the medical industry uses paramagnetic elements in contrast fluid for MRIs.
Diamagnetic Materials
Diamagnetic materials also have a diverse range of uses. Because diamagnetic materials repel magnetic fields, they can be used to protect objects from magnetic forces. Diamagnetic materials are also used to produce MRI machines and electronic components. One cool application is levitation. A material that is heavily diamagnetic can repel so strongly that it levitates! A final fun fact is that water is diamagnetic.
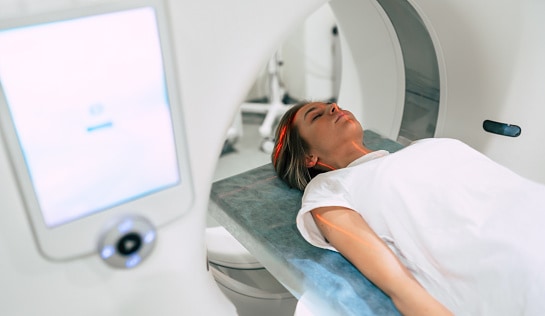
MRIs utilize both paramagnetic and diamagnetic materials.
©Povozniuk/iStock via Getty Images
Conclusion
Understanding the differences between paramagnetism and diamagnetism is an important part of understanding how the world works. Simply put, paramagnetic materials are drawn to the magnetic field, while diamagnetic goods repel it. Looking at the periodic table is a great way to understand which elements are paramagnetic and which are diamagnetic. The distinction primarily relates to electron configuration.
A lot of different industries benefit from each type of magnet. By understanding the way that each type of magnetic property works, you can have a better understanding of why certain elements are used for certain tools and projects!
Thank you for reading! Have some feedback for us? Contact the AZ Animals editorial team.








