While 10th-grade chemistry class may have been a snoozefest, there were valuable pieces of information you may have glazed over. And depending on how long it’s been since you were in high school, you may remember very little. I mean, who really needs to understand allotropes vs. isomers anyway? Just kidding. Stay in school.
Learning about allotropes and isomers can apply to many career fields, from engineering to the pharmaceutical industry. In other words, learning about what they are and the differences between the two can be important, especially if you’re trying to pass 10th-grade chemistry. Discover all there is to know about allotropes vs. isomers, including their key differences, how to identify them, and examples of each.
What is an Allotrope?

As an element goes through a transformation, whether by pressure, temperature, or light exposure, it changes from one allotrope to another.
©Danijela Maksimovic/Shutterstock.com
Allotropes are different forms of the same element. Atoms can bond with one another in different ways, meaning chemical elements can have different structures. The word itself “allotropy,” stems from the Greek word “allotropia,” meaning variability and changeableness.
As an element goes through a transformation, whether altered by pressure, temperature, or light exposure, it changes from one allotrope to another.
And while they are the same element, each allotrope has different properties, both chemical and physical. Each allotrope can look different, feel different, and react differently to temperature and other stimuli.
Most elements have allotropes, but nonmetals are the most well-known.
Examples of Allotropes
Many elements have allotropes, such as carbon, oxygen, phosphorous, tin, arsenic, and iron.
Examples of carbon allotropes include graphite, diamond, fullerenes, graphene, and nanotubules. These allotropes are different structures of the same element (carbon). Even though they are all technically carbon, they differ greatly. For example, diamonds are shiny, strong, and don’t conduct electricity. Graphite is black, can conduct electricity, and is not as very strong.

Examples of carbon allotropes include graphite (above), diamond, fullerenes, graphene, and nanotubules.
©M.M.PHOTO/Shutterstock.com
What Are Allotropes Made of?
Allotropes are different forms of an element. Each allotrope has a different chemical and physical property due to the arrangement of carbon atoms within the structure. Diamonds are crystal structures with their carbon atoms covalently bound to four more carbon atoms. And graphite features rings of six carbon atoms in horizontal sheets.
What Are the Primary Allotropes of Carbon?
- Diamond
- Graphite
- Graphene
- Buckminsterfullerene
What is an Isomer?
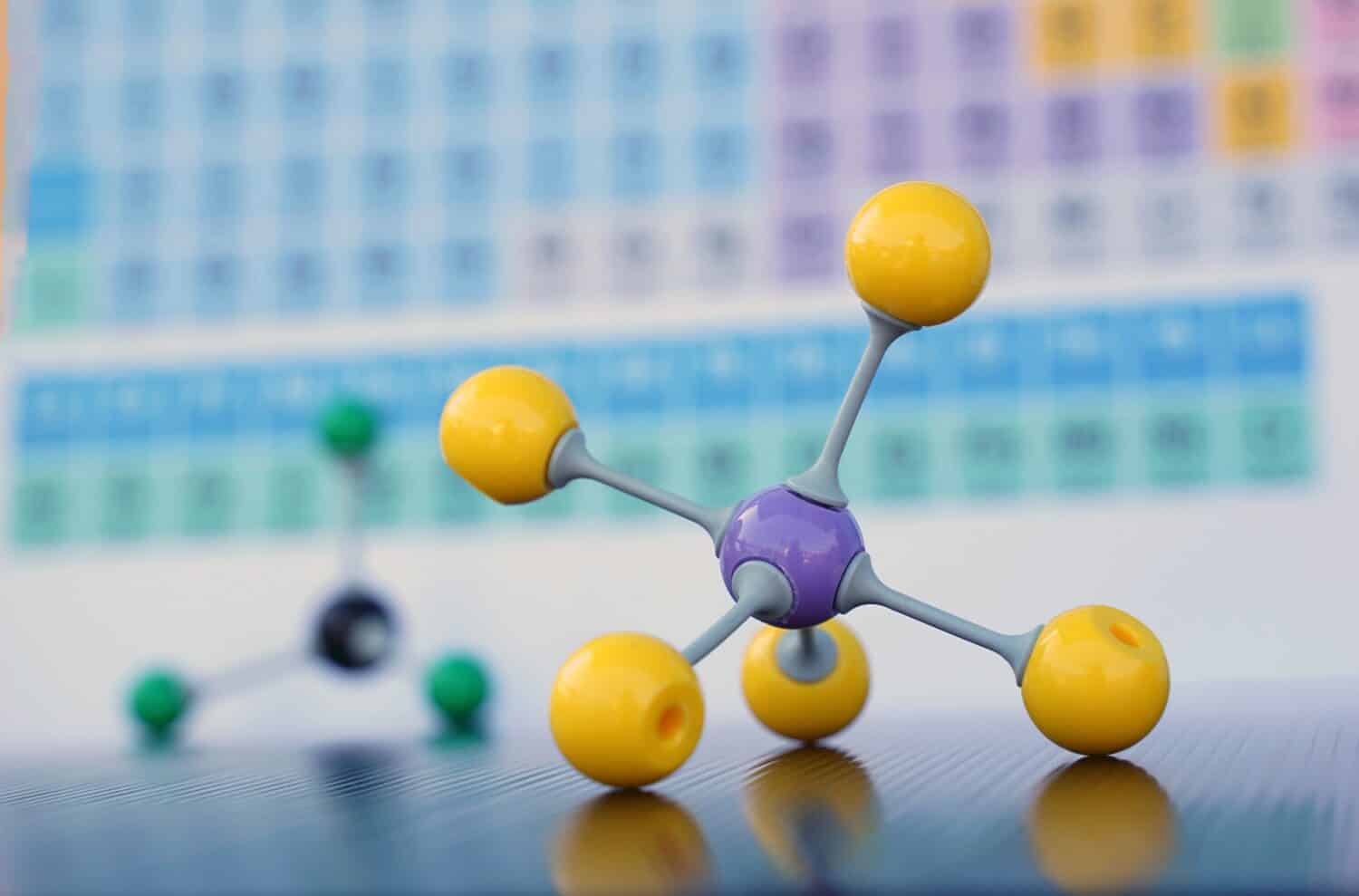
Isomers are chemical compounds with the same parts but are not the same.
©Aoy_Charin/Shutterstock.com
Isomers are molecules that each have the same atoms and the same number of atoms but differ in their chemical properties and physical appearance. Molecules are a group of atoms bonded together. When compounds have the same molecular formula but different structures, they are isomers. In other words, each of these chemical compounds has the same parts but they are not the same.
Types of Isomers
There are two major categories of isomers: structural isomers and stereoisomers. Structural isomers feature the same chemical formula but a different arrangement of atoms. Stereoisomers also have the same chemical formula, and their atomic arrangement is the same too. However, stereoisomers have different spatial arrangements.
Examples of an Isomer
1-Hexane is a clear, colorless, and flammable liquid with a petroleum smell. It has a formula of six carbons and 12 hydrogens and is used in fuels, flavors, perfumes, plastic resins, and dyes.
Cyclohexane is also a colorless, flammable liquid with a formula of six carbons and 12 hydrogens. It has a detergent-like smell and is used to make nylon, paint remover, and other chemicals.
Both contain six carbons and twelve hydrogens, but the structure of their molecules is different. 1-Hexane is a linear chain hydrocarbon, while cyclohexane is a ring hydrocarbon.

1-Hexane and Cyclohexane contain six carbons and twelve hydrogens, but the structure of their molecules is different
©luchschenF/Shutterstock.com
How Are Isomers Used in Everday Life?
Isomers are important in pollution chemistry, nutrition, and the pharmaceutical industry (medicine). Isomerism is essential in developing safer and more effective medicine for either new or existing pharmaceuticals.
Allotropes Vs. Isomers: Key Differences
Use this handy table for a quick glimpse at the differences between allotropes vs. isomers.
| Allotropes | Isomers | |
|---|---|---|
| Definition | Different forms of the same element. | Chemical compounds with the same molecular formula but different chemical and physical properties |
| Atoms | Different amounts of atoms | The same amount of atoms |
| elements | The same element | Different elements |
| Structure | They are always different structures | Can have similar or different structures |
| Example | Diamonds and graphites | 1-Hexane and Cyclohexane |
Thank you for reading! Have some feedback for us? Contact the AZ Animals editorial team.







