While the prevalence of acid rain on Earth has fallen over the last few decades due to the control of causative environmental pollution, it still happens. Sometimes, it occurs in the United States. Is there acid rain in Ohio?
What is Acid Rain?
Acid rain is any acidic precipitation. Usually, the culprit is nitric or sulfuric acid.
While rain usually conjures images of liquid water, acid rain refers to any acidic precipitation. This means hail, snow, falling dust, and fog can also be considered acid rain.
While standard rain is a little acidic, acid rain is more acidic. Expected precipitation contains carbonic acid as a result of dissolved carbon dioxide.
On the pH scale, distilled water has a neutral pH of 7. Any number below 7 means a substance is acidic, and every number lower down the pH scale means acidity has increased by 10.
Standard rain usually has a pH of 5.6. This is almost equivalent to the acidity of human skin. However, acid rain has a pH of about 4.3, making it slightly less acidic than tomatoes.
How Does Acid Rain Happen?
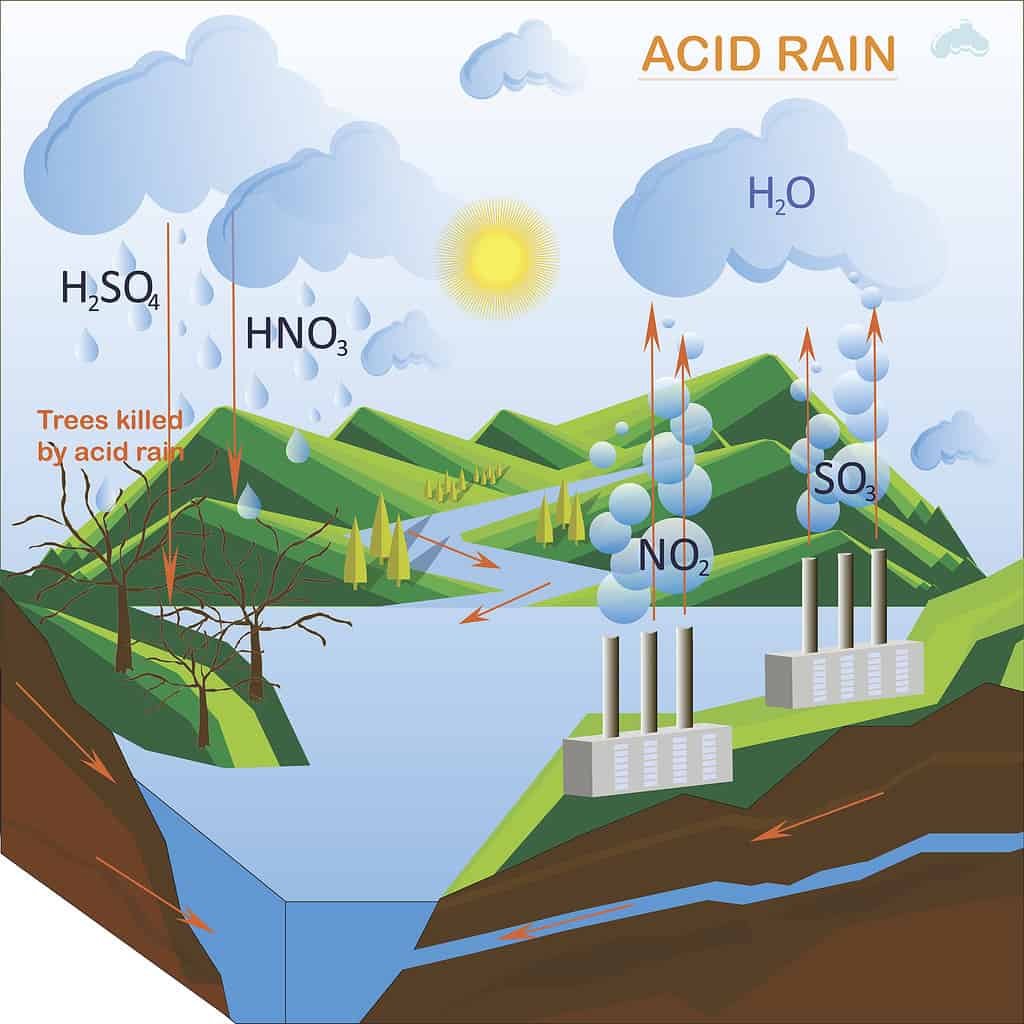
When many chemicals are released into the atmosphere, acid rain can form.
©Danylyukk/iStock via Getty Images
Acid rain happens when chemicals are continually released from a source into the air. This became a problem during the Industrial Revolution and became a prominent ecological concern by the 1960s.
Usually, nitrogen oxide or sulfur dioxide is produced by a power station in huge quantities, which creates plumes of smoke. These plumes climb very high very quickly. Atmospheric winds combine these chemicals with oxygen, water, and other molecules to form nitric and sulfuric acid.
Other artificial sources of acid rain also exist. For example, oil refineries or cities with immense vehicular traffic can create the conditions needed to produce acid rain.
Sometimes, acid rain is caused by volcanic eruptions and other natural phenomena. When an explosion happens, sulfur dioxide is often released into the atmosphere. When this happens, there is a potential for the development of acid rain.
Fact or Fiction: Is There Acid Rain in Ohio?
It is a fact that acid rain has fallen in Ohio.
©iQoncept/Shutterstock.com
Fact. Acid rain has fallen in Ohio, and there’s a slight chance it might fail again within the state.
Before emissions were heavily regulated to curtail acid rain, it occurred frequently in the Midwest and eastern United States. In 1978, rainwater in Ohio had an average pH of 4.2, indicating acid rain. It has steadily improved since then, and today, rain in Ohio averages a normal pH of about 5.6.
At one point before 1990, the Ohio Valley was a significant producer of acid rain. The industry in this region was responsible for more than 50 percent of the acid rain affecting Canadian lakes. This caused an international scuffle that resulted in joint efforts between the US and Canada to improve the kind of pollution causing acid rain.
Ohio: Did Acid Rain Fall in East Palestine in 2023?
On February 3, 2023, a train derailed near East Palestine in Ohio. Thirty-eight train cars derailed, and a massive fire broke out. More than 11 of the rail cars were carrying hazardous substances.
In response to this derailment, authorities conducted a controlled burn of vinyl chloride gas to prevent a catastrophic explosion. Vinyl chloride is a clear carcinogenic gas used to create certain types of plastic. When vinyl chloride is burned, it puts phosgene and hydrochloric acid in the atmosphere. This could technically make rain more acidic.
If acid rain did fall on or around February 3 in Ohio, it would have occurred downwind from East Palestine. However, it’s unlikely that it happened. It didn’t rain in the area for five days post-burn, so the chemicals needed to cause acid rain had dissipated.
Why Is Acid Rain Bad?

Trees are damaged by acid rain.
©iStock.com/Achim Schneider / reisezielinfo.de
Acid rain harms the environment by negatively impacting water sources, animals, and plant life. While one occurrence of acid rain isn’t enough to destroy an ecosystem, the cumulative effects are damaging over time.
When acid rain falls frequently in forested environments, it damages tree bark, making trees vulnerable to pests, temperature extremes, and drought. Soil is degraded because needed nutrients for healthy growth are pulled from it.
Invertebrates are sometimes affected. Organisms like freshwater shrimp are killed because acid rain dissolves their exoskeletons. This causes a cascade effect that harms predators like lake trout that rely on these animals for food.
In cities, acid rain makes paint peel. It also corrodes steel, affecting infrastructure like bridges, and erodes stone, which affects buildings and public statues.
The photo featured at the top of this post is © Whitepointer/iStock via Getty Images
Thank you for reading! Have some feedback for us? Contact the AZ Animals editorial team.







