How weighty is iron, and how do we calculate its mass using modern chemistry? What’s its importance to us as human beings? We’ll define what iron is and discuss why we care about molar mass. Then, we’ll discover the molar mass of iron (Fe) and how it compares to other elements.
What is Iron (Fe)?

Iron is a transition metal that rusts easily when exposed to air or water.
©MMXeon/Shutterstock.com
Iron is a grey shiny metal that is solid at room temperature. Its symbol Fe comes from the Latin word ferrum meaning firmness.
Iron lies within Group 8 on the Periodic Table which is a column filled with the transition metals. Transition metals are malleable, and they conduct electricity. Most of these metals are solid at room temperature except for mercury (Hg).
The transition metals have electrons that fill valence shells except for the outer orbital which is not full. This makes iron, and the other transition metals, prime candidates for the formation of stable ions. This is why almost all natural deposits of iron are found as iron ores.
Iron rusts when exposed to air, and commercial industries go to great lengths to develop products to counteract this. The amount of water in the air affects the rate at which iron rusts. Rust occurs because iron forms ions with other elements which makes it reactive to oxygen in both air and water.
This process wherein iron and oxygen bond is called oxidation, and it produces iron oxide. Iron oxide is the reddish and flaky rust that is widely recognizable on old iron products.
What is Molar Mass?
To understand iron’s molar mass, we must first understand the unit of measurement called a mole. A mole is a special unit of measurement used in chemistry and it gives scientists the ability to understand how much of an element they have.
One mole of iron contains 602 sextillion iron atoms. One sextillion of atoms is a trillion billion atoms. This huge number that defines a mole is called Avogadro’s Number.
Grouping this many atoms allows scientists to use iron in a quantity that is usable in the real world. The mass in grams per mole of elemental iron is iron’s molar mass.
The molar mass of an element is easily identified using the atomic mass of that element on the Periodic Table. The atomic mass of iron is the mass of the iron atom’s nucleic parts.
The parts used to calculate atomic mass are an element’s protons and neutrons. Electrons are not considered when calculating atomic mass because they are so small that their mass is mathematically negligible. Molar mass is a way of inflating the atomic mass of an element so it is usable in scientific applications.
Outside of Iron: Elements With a Molar Mass Exception
There are exceptions to the 1:1 rule that exists between the atomic mass and molar mass numbers on the Periodic Table. The 7 diatomic elements always exist as two bonded atoms of the same element which means their molar mass is twice as large as their atomic mass.
These diatomic elements are hydrogen (H), nitrogen (N), oxygen (O), fluorine (F), chlorine (Cl), bromine (Br), and iodine (I). A few other rare exceptions exist including phosphorus (P) which always presents as a molecule of 4 of its atoms, as well as sulfur (S) and selenium (Se) which present with 8 atoms.
What is the Molar Mass of Iron (Fe)?

The molar mass of iron is 55.845 grams per mole.
©sulit.photos/Shutterstock.com
One mole of iron weighs 55.845 grams, which means its molar mass is 55.845 grams per mole. This number is extrapolated from iron’s defined atomic mass of 55.845 amu as listed in the Periodic Table.
The molar mass of iron isotopes is not the same as the molar mass of the pure element. That’s because the number of neutrons in an isotope is different than in a pure elemental atom. This changes the atomic mass which changes the molar mass.
For example, the isotope iron-54 has a molar mass of 53.94 grams. That’s because it has fewer neutrons than elemental iron which is known as iron-56. Iron-56 is what naturally occurs over 90 percent of the time on our planet.
How Does Iron’s Molar Mass Compare to Other Elements?
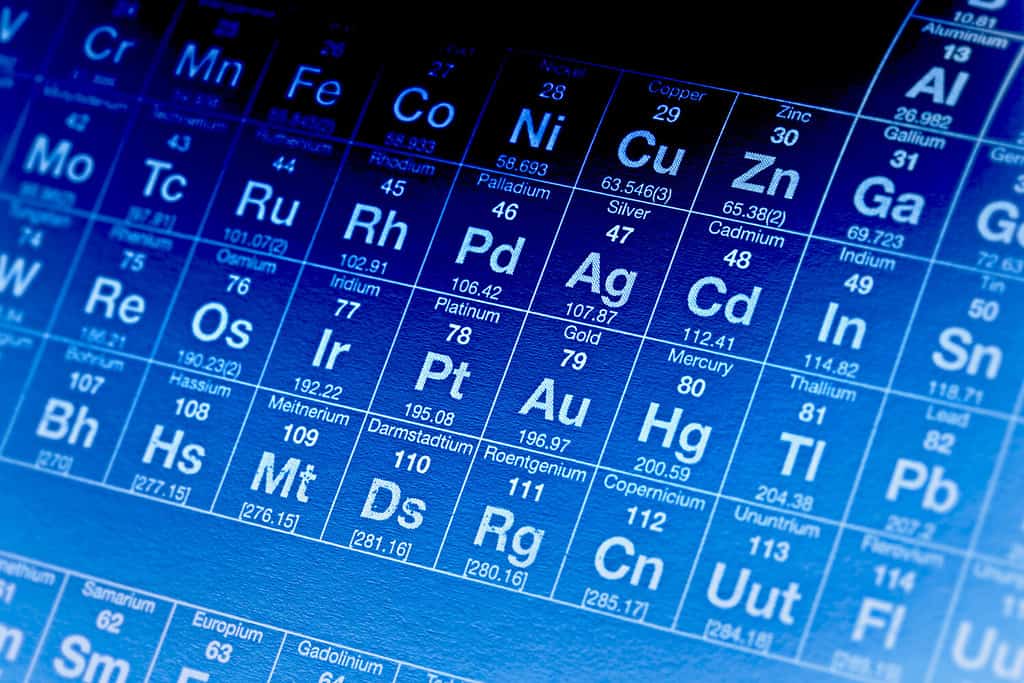
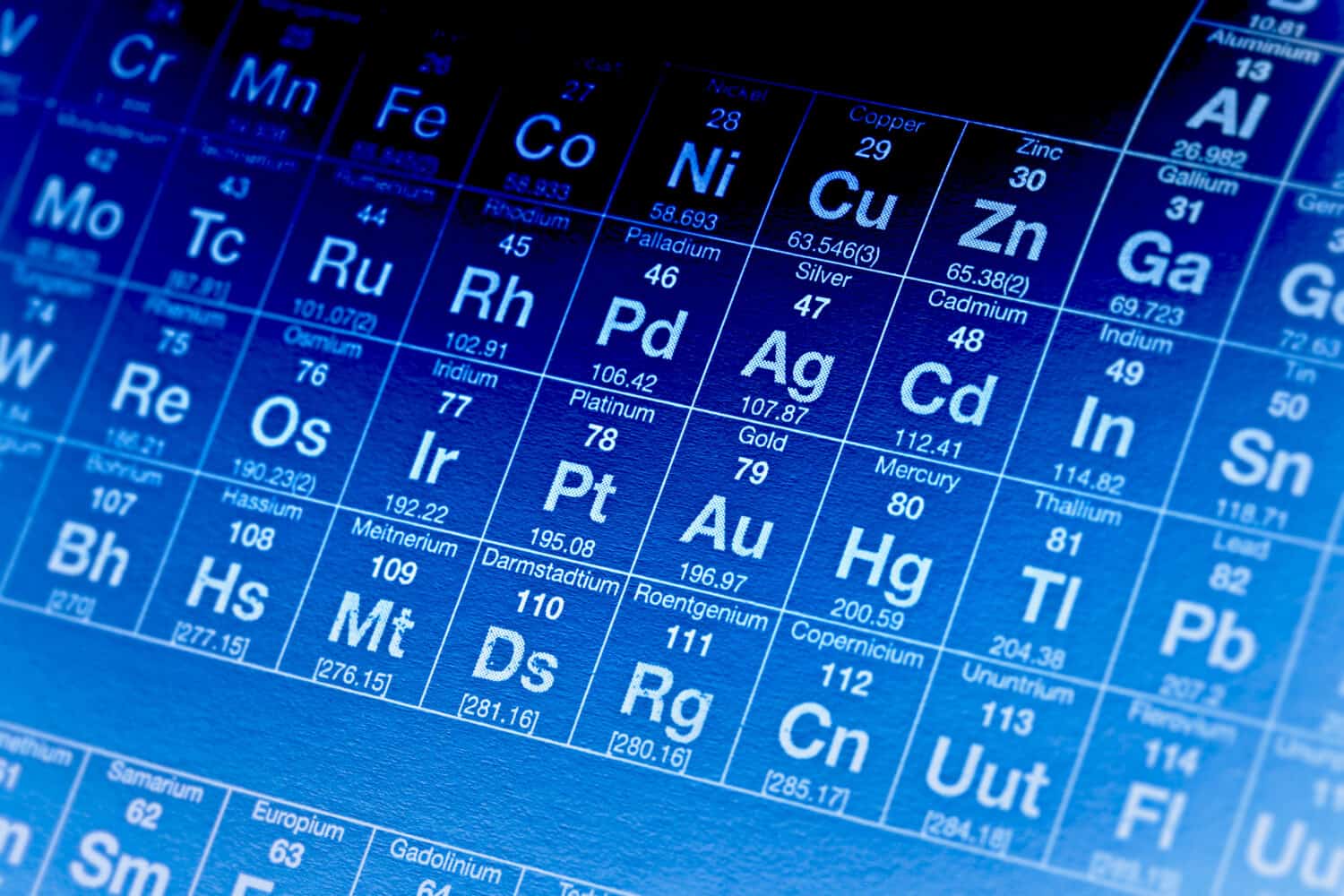
Iron has the twenty-sixth largest molar mass out of 118 elements on the Periodic Table.
©isak55/Shutterstock.com
The atomic mass is also referred to as the atomic weight of an element. It is usually written in the upper lefthand corner of that element’s box on the Periodic Table. Elements on the Periodic Table are organized by atomic mass from left to right and top to bottom.
The same elements on the Periodic Table are also organized by their atomic number. The atomic number defines the number of protons in that element’s nucleus. The more protons in an element’s nucleus, the more mass that atom has.
This starts with 1 in the top left corner at hydrogen and reaches 118 in the bottom right corner with Oganesson (Og). As one travels toward the right of the Periodic Table, and as one travels further down the Periodic Table, atomic mass increases. This means that the element’s molar mass also increases.
Iron’s molar mass is smaller than over three-fourths of the other elements on the Periodic Table. However, there are plenty of elements with a smaller molar mass than iron. Iron has the twenty-sixth largest molar mass out of 118 elements.
Discovering Its Importance: Where Does Iron Occur on Earth?

Humans have manipulated iron since ancient times, and today it’s used mainly in steel production.
©David Tadevosian/Shutterstock.com
Iron is a metal that ancient people learned to manipulate, and this manipulation advanced human civilization. It was such an impactful discovery that there was an entire era of civilization called the Iron Age in honor of the advancements produced by iron’s popularity. While ancient people didn’t understand chemistry, they still used it to manipulate iron ores.
Around 90 percent of the metal that is refined on Earth today is iron as it’s used to create steel. Steel is created by combining iron and carbon in extreme heat. The temperatures used during metal casting hover around 2600 degrees Fahrenheit.
Iron is the most common element found on Earth, and it is the tenth most common element in the universe. It is also the fourth most common element in Earth’s crust. Over 5 and a half percent of the Earth’s crust is made of iron.
Iron’s existence in the human body is extremely important. At any given time, the average person has 4 grams of iron in their body. A large portion of the body’s iron is in the hemoglobin of red blood cells.
Thank you for reading! Have some feedback for us? Contact the AZ Animals editorial team.