Let’s face it, chemistry is one of the hardest academic fields to master. It can sometimes be hard to even understand the very basics of it! One reason why it is so challenging is that it is material that is not often discussed in everyday life. Additionally, a lot of it is diverse and requires some good memorization and conceptual work to understand.
One of the most challenging concepts is functional groups, which form the basis of a lot of chemical reactions. Two groups that particularly pose a challenge for students are amines and amides. Although both have a similar nitrogen component, they have important differences that are important to their chemistry. Don’t worry if you’re lost on the differences between them. Today, we will discuss the great debate of amine vs. amide.
What Are Amines?
Amines are a special function group in organic chemicals that consists of a signature nitrogen. Amines are classified as a nitrogen attached to some carbon chain. Likewise, depending on how many carbons are present, they may also have attached hydrogens. Amines are considered to be primary, secondary, or tertiary, depending on if they have one, two, or three attached carbons respectively.
Amines also aren’t constrained to carbon chains, as they can be found within carbon rings, or even attached to the ring as a side group. Similar to oxygen and hydrogen, the nitrogen in amines can exhibit some polarity. Take note, due to the electronegativity, it isn’t nearly as strong as the polar attraction in an oxygenic compound like water. Additionally, amines can bond with a maximum of three molecules. They always leave a lone pair of electrons in one of their orbitals.
Amine Reactivity
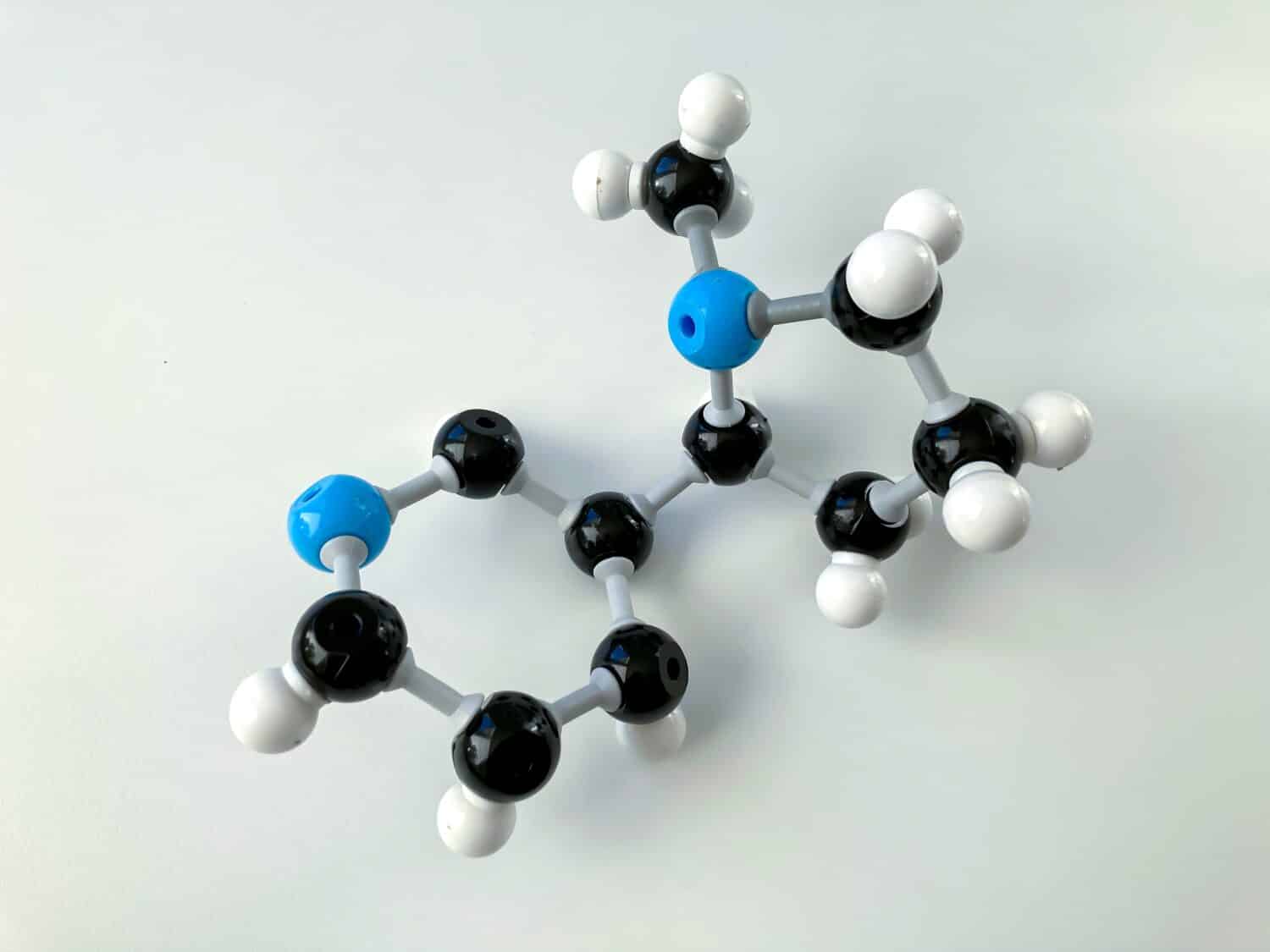
Like other molecules, the main structure that impacts reactivity is the electronegative atom in amines!
©Elzbieta Krzysztof/Shutterstock.com
As touched upon, amines do show some polarity, which can form some weak bonds and reactions. These attractive forces between molecules without the formation of true bonds are called intermolecular forces.
In the case of amines, these forces can take the form of dipole-dipole and ion-dipole. In other words, the somewhat polar group of nitrogen can interact with other polar molecules, or with ions such as salts. Additionally, depending on the size of the amine, it can also have dispersion force interactions, which increase with molecular size and surface areas.
Another important reactive note about amines has to do with acid and base chemistry. The lone pair of electrons that amines carry makes them decent bases. When interacting with acids that have hydrogens, the lone pair can pluck them off. With that said, amines are still very weak bases, meaning they have a limit to their reactivity.
Production of Amines
Even though amines widely occur in nature, there are a few chemists who have devised ways to produce them in the lab. As you can probably guess, to make an amine, you must attach some sort of nitrogenous group to some sort of carbon chain. The base chemistry of amines can aid in this process, as they readily react with acids, which often consist of carbon groups. Thus, the most common way in which amines are formed is through the reaction of ammonia and an acid. These reactions are often done under high heat to reduce the formation of water. Metal catalysts are also commonplace in these reactions.
Naturally Occurring Amines
Amines are extremely common in nature, specifically in the plant kingdom. They compose a wide variety of natural molecules, most of which are described as alkaloids. For instance, plant alkaloids can function in cell maintenance, cellular defense, and even pigment production. Humans can even derive alkaloids from plants for use in products such as medicines. Some amines can be found in animal systems as well, although their function is usually thought to be more generalized in cell maintenance.
In addition to these functions, amines also play important roles in a lot of natural processes. For instance, a lot of animal neurotransmitters consist of amine groups, which sometimes function in their signaling ability. In other words, the qualities of amines lend them to perform in various reactions that naturally occur.
What Are Amides?
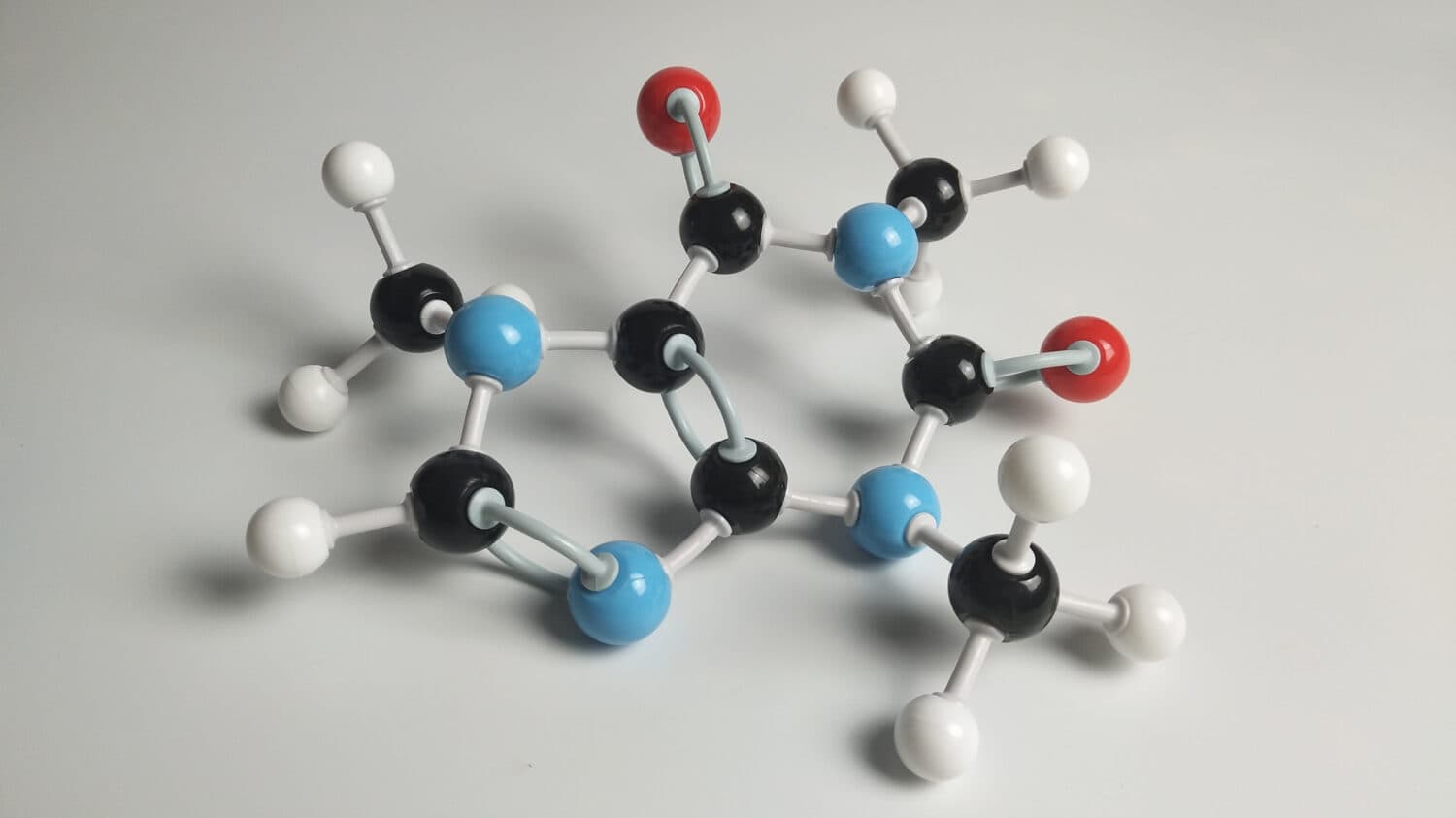
The added oxygen present in amide groups yields more electronegative activity.
©Mr.Yankittaphak Phoyalo/Shutterstock.com
As compared to amines, amides are slightly more complicated in structure and nature. For a general rule of thumb, amides are usually derived from amines. This may help you remember that the common thing between them is nitrogen, as that is all an amine has to offer in a reaction. The main thing that separates amides from amines is the presence of a carbonyl group. If you are not too familiar with carbonyl groups, they are characterized by the double bonding of an oxygen to a carbon. They can be found commonly in carbon chains, or other functional groups like carboxylic acids. Due to the presence of both a carbonyl group and nitrogen, amides look like a hybrid between carboxylic acids and amines.
Amide Reactivity
Just like amines, amides have a fair amount of polarity due to the presence of electronegative nitrogen and oxygen atoms. This means that they will interact with ions and polar molecules, and will readily dissolve in polar solvents such as water. Just like amines, they exhibit similar intermolecular forces, such as ion-dipole and dipole-dipole interactions. Furthermore, they will also show some dispersion force interactions, especially if they are a part of large molecules. In essence, the intermolecular forces of an amine vs. amide are relatively similar.
They lie between amines and carboxylic acids in terms of reactivity as well. In other words, they don’t like to act as bases. They also don’t give up hydrogen atoms as easily as carboxylic acids. This makes them useful for specific chemical applications, such as the creation of ionized drugs that are meant to readily dissolve in natural solvents.
Production of Amides
As mentioned, amides can be produced with amines as a reactant. There are two main methods in which amides are produced. The first involves the addition of a carboxylic acid and an amine group. This also usually requires other solvents to help facilitate the reaction properly. Additionally, amines can also be produced through the addition of an acyl chloride with an amine group. In the end, thinking of the separate components that form amides can help in understanding what is needed to produce them!
Naturally Occurring Amides
Similar to amines, amides are also very common in biological systems. For instance, they can form the base of many well-known molecules, such as vitamin B. Likewise, they are commonly used in animals to divert nitrogenous waste out of the body as urea.
In addition to these novel molecules, amides are similar to amines in the fact that they can also form molecules that play important natural functions. On top of neurotransmitters, amides can also be a part of other signals, such as epinephrine and histamine.
Amine vs. Amide
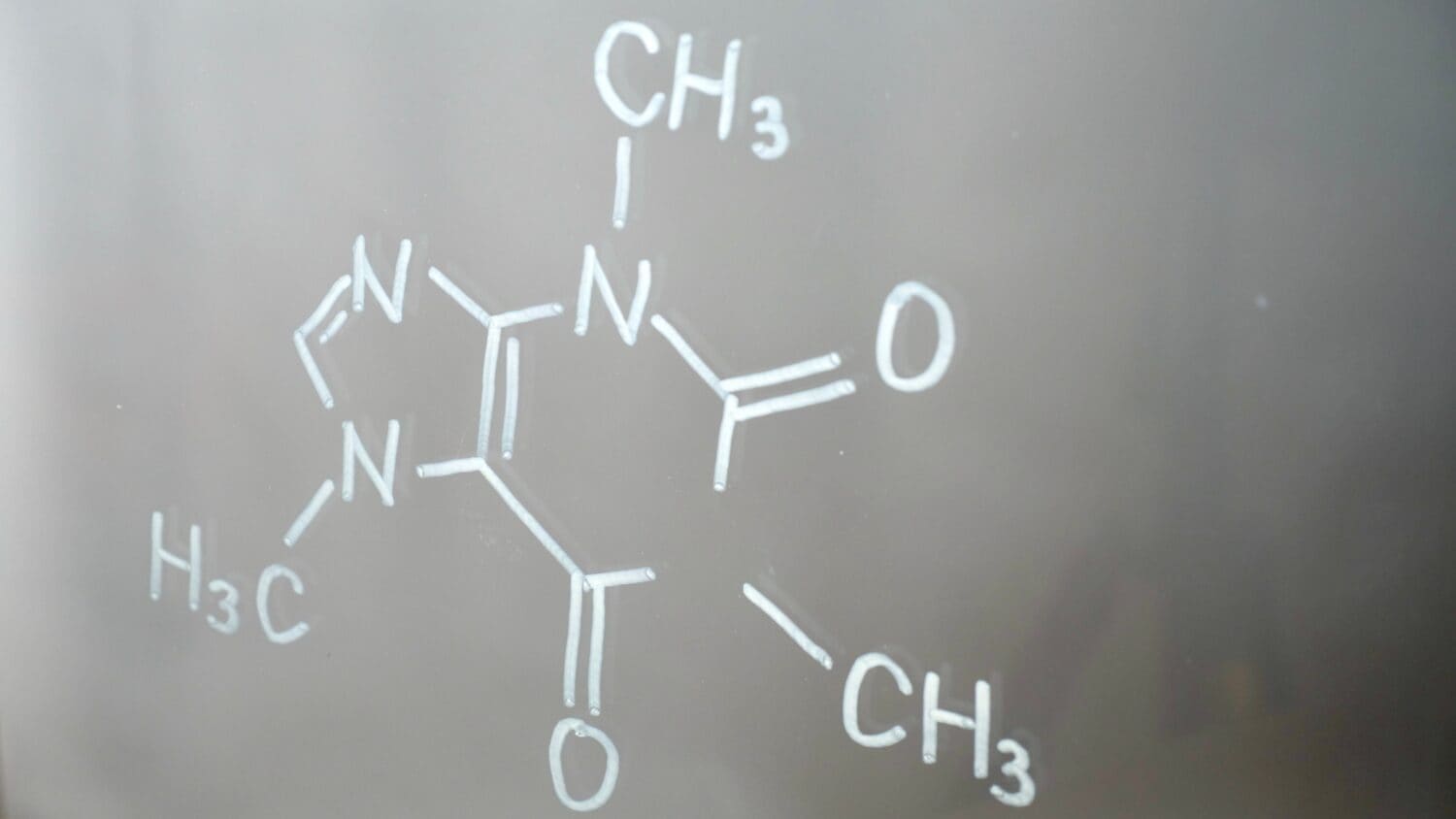
Many groups can be present within one chemical. This means amides and amides can be a part of one entire structure!
©OksanaFedorchuk/Shutterstock.com
As you have probably collected from this article, there are a lot of differences to understand between amines and amides. To help summarize, take a look at the bullets below that list the main differences between an amine vs. amide.
- Amines are formed from the presence of nitrogen, while amides require the additional presence of an attached carbonyl group
- Amines can function as weak bases and are derived from ammonia.
- Amides can function as extremely weak acids and are derived from amines and acids.
The photo featured at the top of this post is © Love Employee/iStock via Getty Images
Thank you for reading! Have some feedback for us? Contact the AZ Animals editorial team.







