Carbon is a common element in the universe that has a unique mass like all of the other elements on the Periodic Table. What is that mass, and how do we measure it? We’ll discover the molar mass of carbon (C) and how it compares to other elements now.
What Is Carbon?

Carbon creates diamonds, graphite, and anthracite.
©RTimages/Shutterstock.com
Carbon is an element that’s solid at room temperature, and it’s one of the most important elements on the planet. It’s a mandatory requirement in many biological processes and inorganic compounds. You’ll find carbon in some quantity in all living things.
Two of the most recognizable forms of carbon include diamonds and graphite. It also naturally occurs as a type of coal named anthracite.
Diamonds exist within kimberlite. Kimberlite is a rock present in vertical streaks in the planet’s crust. There are active diamond mines in South Africa, Botswana, the Democratic Republic of Congo, Russia, and Canada.
What Is a Molar Mass?
There are 6.022 x 10
23atoms or molecules in one mole.
©Dabarti CGI/Shutterstock.com
One mole of a substance has a mass, and this mass is the molar mass. Molar masses are determined by adding up the atomic masses of all of the elements in a compound, and these atomic masses are listed on the periodic table.
A mole contains 6.022 x 1023 atoms or compounds. This means that the molar mass is the total mass of all compounds or standalone elemental atoms in a mole. Molar mass is usually expressed in grams.
Using a mole as a unit of measurement is necessary for meaningful experiments in laboratory environments. That’s because it allows for a usable size of the element or compound without needing special equipment that can manipulate a single atom or molecule. It makes something microscopic and hard to assess a macroscopic lump sum.
How to Calculate Molar Mass
To calculate molar mass, divide the number of grams of a substance by the number of moles present. The following equation represents this:
Molar mass = (mass in grams) / (number of moles)
Keep in mind that any single mole of any substance always has the same number of units in it. Additionally, the atomic mass of an element on the periodic table is numerically equivalent to the molar mass of that element. The atomic mass is much smaller in reality as it’s measured in atomic mass units that reference one atom; the molar mass refers to all of the grams in a mole.
What Is the Molar Mass of Carbon?

The molar mass of carbon is 12.0107 grams/mole, and scientists often round it to simplify calculations.
©Danijela Maksimovic/Shutterstock.com
The molar mass of carbon is 12.0107 grams/mole. In most simple calculations, the molar mass of carbon is defined as 12 grams/mole. Carbon is an element, so 6.022 x 1023 atoms of carbon equals one mole of carbon.
Why Carbon Is Important

Carbon is essential to life, and carbon dioxide is an essential molecule of carbon.
©peterschreiber.media/ via Getty Images
Carbon is important because it is one of the elements that are essential to life on Earth. It easily combines with oxygen to form carbon dioxide (CO2). Carbon dioxide helps create carbohydrates, which are essential to the formation of RNA, DNA, and amino acids.
Carbon is capable of forming strong chains in many different situations with the help of hydrogen atoms. These chains, like carbohydrates, are called hydrocarbons.
Hydrocarbons make up most of the compounds that create natural gas, coal, and oil. These products are invaluable to modern life as they are our primary fuel source in today’s society.
As a consequence of industrialization, carbon dioxide is driving climate change. That’s because as fossil fuels are burned, carbon emissions are released into the air. This traps heat within Earth’s atmosphere. Attempts to replace fossil fuels with green energy that doesn’t create excess carbon dioxide include nuclear energy, hydropower, wind energy, and solar energy.
Elements Close in Molar Mass to Carbon
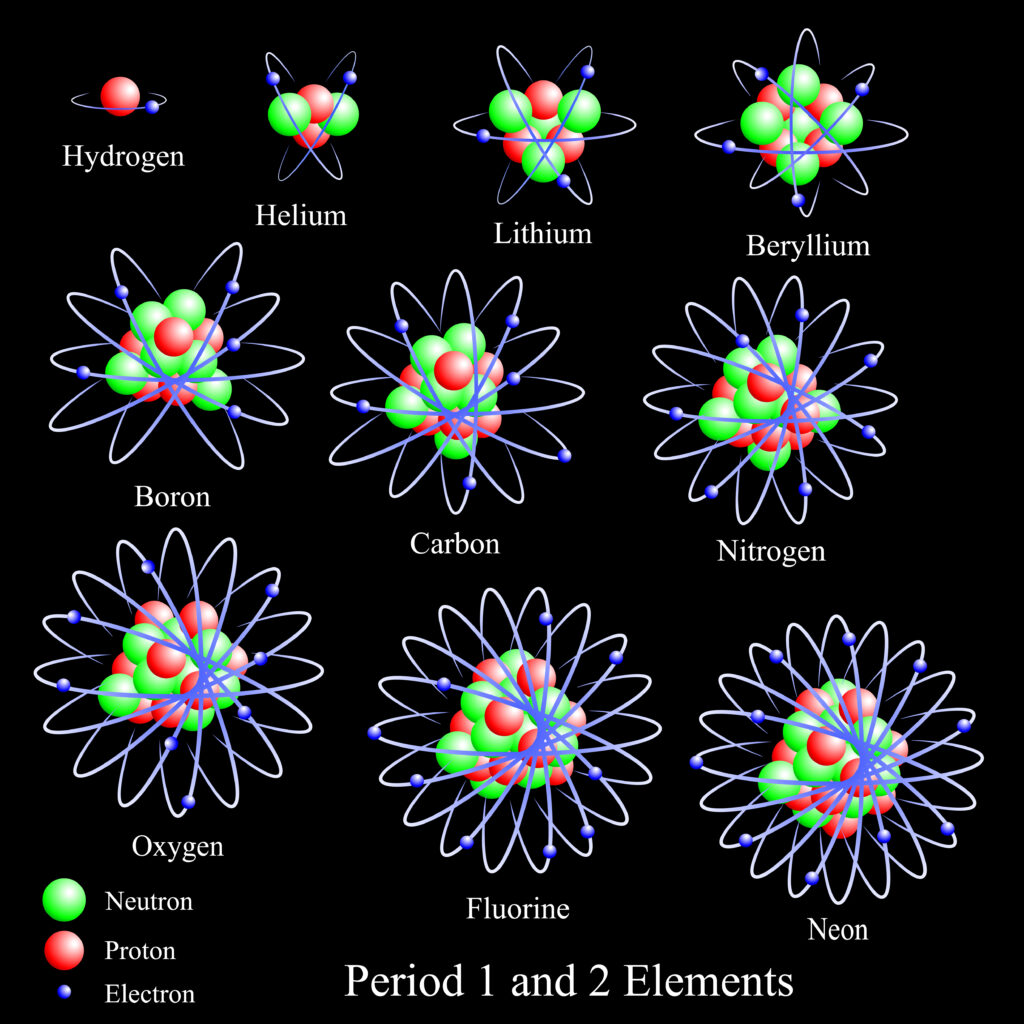
The number of protons and neutrons in an element defines its atomic mass and molar mass.
©Mike Price/Shutterstock.com
Every element has a different molar mass because they have a unique number of protons and neutrons. While electrons are the third component of the anatomy of an atom, their weight is so negligible that they aren’t important when calculating mass.
Elements close in molar mass to carbon include:
- Beryllium (Be): 9.0122 grams/mole
- Boron (B): 10.81 grams/mole
- Nitrogen (N): 14.007 grams/mole
- Oxygen (O): 15.999 grams/mole
Carbon has six protons and six neutrons which gives it a molar mass of twelve grams/mole. Beryllium, which is lighter, only has four protons and four neutrons which is why it has a molar mass of nine grams/mole. Boron is also lighter than carbon because it contains five protons and five neutrons.
Two more elements with close molar masses to carbon are nitrogen and oxygen. They’re both more massive than carbon because they have more protons and neutrons than carbon. Nitrogen has seven protons and seven neutrons; oxygen has eight protons and eight neutrons.
Summary
- Carbon is essential to everything alive on Earth and without it, life wouldn’t exist.
- Carbon is also a vital element in many inorganic compounds.
- Molar mass exists as a macroscopic measurement, which allows scientists to do experiments without expensive equipment that manipulates single atoms.
- A mole is equal to the mass of 6.022 x 1023 atoms or molecules in a substance.
- The molar mass of carbon is 12.0107 grams/mole. This is often rounded down to 12 grams/mole.
- Carbon emissions created by human industry are driving climate change.
The photo featured at the top of this post is © Ekaterina79/iStock via Getty Images
Thank you for reading! Have some feedback for us? Contact the AZ Animals editorial team.







